Root Cause Analysis VS FMEA

RCA VS FMEA
- When we speak of “quality” in health care, we generally think of mortality outcomes or regulatory requirements mandated by the JCAHO (Joint Commission for Accreditation of Healthcare Organizations).
- But how do these relate to and impact our everyday lives as hospitalists?
- The workshop starts by stepping into the life of a hospitalist and something we all fear: “Something terrible happens.
- Then what?” Depending on the event’s severity, the options include peer review, notifying the Department Chief, calling the Risk Manager, calling your lawyer, or doing nothing.
- You’ve probably had many experiences when “something wasn’t quite right,” but often there is no apparent bad outcome or obvious solution, so we shrug our shoulders and say, “Oh well, we got lucky this time; no harm, no foul.”
- The problem is there are recurring patterns to these events, and the same issues may affect the next patient, who may not be so lucky.
Defining “Something Bad”
- These cases, which have outcomes ranging from no effect on the patient to death, may be approached in several different ways.
- The terms “near miss” or “close call” refer to an incident where a mistake was made but caught in time, so no harm was done to the patient.
- An example is when a physician makes a mistake on a medication order, but it is caught and corrected by a pharmacist or nurse.
- When adverse outcomes occur, consider and define ethologies to identify and address underlying causes.
- Is the outcome an expected or unexpected complication of therapy? Was there an error involved? In asking these questions, remember that you can have harm without error and error without liability.
- This definition points out that usually, a chain of events rather than a single individual or event results in a bad outcome.
- Significant adverse events are called “sentinel events” and are defined as an “unexpected occurrence involving death or serious physical or psychological injury, or the risk thereof. Serious injury specifically includes loss of limb or function” (JCAHO 1998).
How We Approach Error
- Unfortunately, we are fallible and make errors quite reliably as humans.
- For example, we make errors of omission 0.01% of the time, but the good news is that with reminders or ticklers, we can reduce this rate to 0.003%.
- Unfortunately, when humans are under high stress and in danger, research from the military indicates error rates of 25% (Salvendy 1997).
- In a complex ICU setting, researchers have documented an average of 178 activities per patient per day with an error rate of 0.95%.
-
The reality is that we err.
- Having the unrealistic expectations developed in medical training of being perfect in all our actions perpetuate the blame cycle when the inevitable mistake occurs, and it prevents us from implementing solutions that prevent errors from ever happening or catching them before they cause harm.
- RCA and FMEA Help Us Create Solutions That Make a Difference.
What is Root Cause Analysis
- Briefly, Root Cause Analysis (RCA) is a retrospective investigation that JCAHO requires after a sentinel event: “Root cause analysis is a process for identifying the basic or causal factor(s) that underlies variation in performance, including the occurrence or possible occurrence of a sentinel event. A root cause is the most fundamental reason a problem―a situation where performance does not meet expectations―has occurred” (JCAHO 1998).
- An Root Cause Analysis looks back at an event and asks, “What happened?”
- The utility of this methodology lies in the fact that it not only asks what happened.
- However, asks, “Why did this happen” rather than focus on “Who is to blame?”
- Some hospitals use this methodology for cases that are not sentinel events.
- Because the knowledge gained from these investigations often uncovers system issues previously unknown.
- In addition, it negatively impacts many departments, not just those involved in a particular case.
Case Illustrations
Case Example 1
- A 42-year-old primigravida female at 34 weeks gestation was brought to the obstetric emergency at midnight with complaints of severe headache, blurry vision, and right upper quadrant pain for the last five to six hours.
- The patient noted gradually increasing lower extremity oedema and facial swelling. She has a history of gestational hypertension and was prescribed labetalol 200 mg twice a day one week previously.
- At the time of presentation, the blood pressure was 190/110 mm Hg on two separate occasions, five minutes apart.
- She had gained two kilograms since her last antenatal checkup in the clinic one week previously.
What about the diagnosis?
- The patient was diagnosed with severe pre-eclampsia. The senior obstetric resident ordered a loading dose of magnesium sulfate to prevent imminent seizure.
- In the hospital protocol used, the IV and IM regimen where the patient receives a four-gram (20% concentration) intravenous solution bolus and a 10-gram intramuscular dose (50% concentration), five grams in each buttock.
- For the senior resident provided the order for magnesium sulfate administration to the junior resident verbally, who subsequently verbally communicated the order to the nurse.
- This magnesium sulfate dosing regimen is complex, with multiple doses in different locations, and was incorrectly prepared by the nurse who felt rushed in an urgent situation.
What is displayed in the chart?
- A chart displaying magnesium sulfate’s preparation in the drug preparation room had faded and was supposed to be replaced but was delayed.
- Therefore, the nurse prepared the medication relying on her memory.
- Before administering the medicine to the patient, as a part of the protocol, she repeated the dose strength aloud to another nurse, who cross-checked it from a printed chart and picked up the error in time.
- The senior resident also identified the error as the dose was communicated aloud and stopped administering the drug.
-
Magnesium sulfate is on the Institute of Safe Medication Practices’ list of high-alert medications.
- The drug has a severe risk of causing significant patient harm when used in incorrect dosages and concentrations.
- Accidents and adverse outcomes continue to occur with magnesium sulfate in obstetrics because of the complex dosing regimen and preparation.
- It is worthwhile to review essential safety procedures that can minimise risk. It is advisable to use premixed solutions prepared by the pharmacy for the bolus rather than requiring nurses to mix high-risk medications on the unit.
Learning lesson:
- Variation is an inherent part of each process and contributes to errors in medicine.
- By standardising the activities in each process, variation can be minimised, and errors can be reduced.
- Before administering high-risk medications, a second nurse should double-check all doses, pump settings, the drug name, and concentration should be read out loud in front of the care delivery team.
Case Example 2
- The name and date of birth used in this example are imaginative, used for illustrative purposes, and do not represent an actual patient.
- Any similarities, if noted, are purely coincidental, considering that there are more than 7 billion people worldwide!
- Anna Joy (date of birth October 30, 1991) was admitted to a busy obstetric ward.
- She was a primigravida at 30 weeks of gestation with complaints of intermittent cramping abdominal pain.
- She had come to visit her sister living in Boston from Spain.
- The patient’s ability to communicate in English was limited, and she preferred speaking Spanish.
- However, her husband and sister were fluent in English and assisted with translation throughout the history, exam, and admission.
- The patient was seen by an obstetrician who advised routine investigations for threatened preterm labour and observation.
-
Another patient Ann Jay (date of birth September 30, 1991), was also admitted to the same day’s obstetric ward.
- She was 34 weeks gestation and was admitted because of gestational diabetes mellitus with hyperglycemia.
- Her obstetrician advised an endocrinology referral, and the endocrinologist advised glucose monitoring and insulin administration.
- Nurse taking care of the patient was provided with the instructions and performed a finger-stick blood glucose check.
- Additionally, I informed the endocrinologist about the results over the phone.
-
The endocrinologist advised six units of regular insulin pre-lunch.
- Then, the nurse also informed the obstetrician that the patient felt a decrease in fetal movements. The obstetrician advised ongoing observation, and fetal kick counts.
- The family members of the first patient, Anna Joy, informed the nurse that they were going to lunch.
- In the morning shift nurse took a half-day leave because of personal issues and quickly handed over the patient to another nurse. The ward was busy and running at total capacity.
-
The new nurse decided to administer the insulin injection as the patient was waiting for lunch.
- She did not know that Anna Joy preferred communication in Spanish and family members were absent during that time.
- The nurse asked a few questions and rushed through patient identification with the help of two unique patient identifiers.
- She administered the insulin injection to the first patient and later realised that it was supposed to be given to the second patient, Ann Jay.
- The attending obstetrician of the patient and the endocrinologist were informed.
- They took the necessary measures and closely monitored the patient for the next few hours.
-
No inadvertent effects were noted.
- The nurse caring for both patients has worked in the hospital for five years and was recently transitioned to the obstetric ward.
- This had never happened to her before, and she realised that she should have checked the instructions more carefully when setting up the patient’s medication.
- She thought she performed the patient identification information, but not carefully enough.
- She did not check the patient’s armband and could not communicate effectively with the patient because of the language barrier. Also, the system relied on using the patient’s family members and not hospital interpreters for communication.
- The hospital procedure for verifying patient identification information was using two unique patient identifiers, the name and the date of birth.
- During the handover process between the two nurses, there was no highlighting that the patients had similar names and dates of birth.
Learning lesson:
- The modern patient care delivery process relies not on an autonomous physician but efficient and effective integration of a multidisciplinary care provider team.
- Additionally, the team comprises clinicians from different sub-specialities, nursing staff, and other allied healthcare professionals.
- A clear, consistent, and standardised communication method between the team members contributes to the firm foundation of patient care.
- During shift change, the hand-off between clinicians and nurses is pivotal in providing high-quality care.
- The aim should be to provide up-to-date, accurate, and complete information to the oncoming team. The care providers need education about the importance of effective hand-offs.
- Similar-sounding patient names can result in significant medical errors. Two unique identifiers should always be used with each interaction with a patient.
Case Example 3
- A 26-year-old primigravida female was admitted to a busy hospital’s labour and delivery suite at 39 weeks gestation with labour pains.
- There were no associated high-risk factors.
- The patient was admitted to the labour ward and managed per the routing protocol. She progressed in spontaneous labour, but the cardiotocograph showed prolonged fetal bradycardia lasting for three and a half minutes at 4 centimetres (cm) cervical dilatation.
- The fetal bradycardia did not resolve with initial conservative measures.
- The patient was transferred to the operating room for a category one emergent cesarean section.
- A category one cesarean section means the baby should be delivered within 30 minutes of the procedure’s decision. It is done when there is an immediate threat to the life of the mother or the baby.
-
The baby was delivered in good condition, and there were no intraoperative complications.
- However, before closure, the operating obstetrician asked the scrub nurse to perform a surgical count.
- The scrub nurse reported a missing gauze piece from the surgical trolley. The count was performed several times by the scrub and the floor nurse.
- The second on-call obstetrician was called to assist the primary surgeon in checking for the surgical field’s missing gauze piece.
- The surgical gauze had a heat-bonded barium sulfate marker embedded in the fabric to assist with x-ray identification.
- An intra-operative x-ray was obtained to evaluate for intra-peritoneal gauze and was negative. The department chief discussed the case, and abdominal closure was performed.
- Due to the associated delays, the operative time was increased significantly (two hours and thirty minutes).
Learning lesson:
- The surgical count is critical and must be performed in a standardised fashion that eliminates variation and error.
- Per international standards, there should be standardisation of the counting process and systematic tracking of the instruments, gauze, and sponges in the sterile field.
- To eliminate errors, the counting process should be concurrently audible and visual.
- The process should be performed between the scrub nurse and the circulating nurse. The best practices for surgical count should always be followed.
Case Example 4
- A 25-year-old male presented for bilateral LASIK surgery at a same-day surgery centre.
- The operating surgeon examined the patient, a community-based surgeon who does not routinely operate at this facility. Informed consent was obtained by the functional surgery pre-operatively.
- The refractive error was -4 D for the right eye and – 5D for the left eye. The plan was to eradicate the refractive error. There was a timeout to ensure the correct patient and procedure.
- The LASIK was started by making corneal flaps on both eyes, completed uneventfully. The second step was the excimer laser-guided corneal power correction.
- The patient was adjusted on the operating microscope so that the first eye was directly under the excimer laser, and iris recognition was attempted.
-
The machine did not recognise the iris pattern after three attempts.
- The surgeon decided to proceed without iris recognition.
- The technician thought that this was rare and that they had reasonable iris recognition rates for this month (> 98%). However, he did not want to contradict the surgeon, who was known for his temper!
- Before the procedure, the circulating nurse, noted that the patient’s table was adjusted to the wrong side, and the left eye was under the laser instead of the right.
- She pressed the emergency stop button, and the treatment was terminated. After identifying the mistake, the surgeon and technician restarted the machine to treat the correct sequence’s right eye.
- Compared to unilateral procedures, bilateral procedures are incredibly challenging, mainly if the treatment varies between the two sides.
- An example is LASIK, where both eyes are typically corrected simultaneously, and there is no obvious pathology on the eye except for the refractive error.
-
The correction is determined preoperatively, and the result is not immediately titrated.
- There is a significant chance for wrong-site procedures, given these ambiguities.
- To avoid this disaster, a Swiss cheese-type pattern is implemented in LASIK centres where the optometrist and technician are.
- Additionally, the surgeon always verifies each eye’s refractive error before the procedure and after programming the laser.
- Some advanced laser machines have an inbuilt layer of defence where the iris pattern of the eye is uniquely identified via iris recognition, which helps determine the correct look and enhances the treatment fidelity.
- Some treatments do not include iris recognition; therefore, the onus lies on the surgeon to correctly identify the right eye.
Learning lesson:
- The Swiss cheese model works on the principle that if there are multiple layers of check between the planned event and its execution, the error is preventable.
- This draws on an analogy from layers of swiss cheese stacked together. Each slice of cheese represents a checkpoint to avoid error.
- The holes in the cheese slices are random, representing an unexpected error.
- However, if multiple layers are stacked together, the probability of missing the error decreases.
- As noted in this example, rechecking the right site for the proper treatment dose can avoid disasters in bilateral procedures, especially when there are no obvious differentiating pathologies on examination.
What is Failure Modes and Effects Analysis (FMEA)?
- Failure Modes and Effects Analysis (FMEA) is a prospective investigation aimed at identifying vulnerabilities and preventing failures in the future.
- It looks forward and asks what could go wrong.
- Performance of an FMEA is also required yearly by JCAHO and focuses on improving risky processes such as blood transfusions, chemotherapy, and other high-risk medications.
- Approaching a clinical case demonstrates the differences between Root Cause Analysis (RCA) and FMEA.
- Imagine a 72-year-old patient admitted to your hospital with findings of an acute abdomen requiring surgery.
-
The patient is a smoker with Type 2 diabetes and an admission blood sugar of 465 but no evidence of DKA.
- She usually takes an oral hypoglycemic to control her diabetes and an ACE inhibitor for high blood pressure but no other medications.
- She is taken to the OR emergently, where surgery seems to go well, and post-operatively, she is admitted to the ICU. Subsequently, her blood glucose ranges from 260 to 370 and is “controlled” with sliding-scale insulin.
- Unfortunately, within 18 hours of surgery, she suffers an MI and develops a postoperative wound infection four days after surgery.
-
She eventually dies from sepsis.
- The Root Cause Analysis (RCA) of this case might reveal causal factors such as lack of use of a beta-blocker preoperatively and lack of use of IV insulin to lower her blood sugars to the 80–110 range.
- While possibly identifying the root cause of this adverse outcome, an RCA is limited by its hindsight bias and the labour-intensive nature of the investigation that may or may not have broad application since it is an in-depth study of one case.
- However, Root Cause Analysis (RCA) have the beneficial effects of building teamwork, identifying needed changes, and, if carried out impartially without assigning blame, can facilitate a culture of patient safety.
- FMEA takes a different approach and proactively aims to prevent failure.
- It is a systematic method of identifying and preventing product and process failures before they occur.
-
It does not require a specific case or adverse event.
- Rather, a high-risk process is chosen for study, and an interdisciplinary team asks, “What can go wrong with this process, and how can we prevent failures?”
- Considering the above case, imagine that before it occurred to you.
- As the hospitalist concerned with patient safety decided to conduct an FMEA on controlling blood sugar in the ICU or administering beta-blockers perioperatively to patients who are appropriate candidates.
- For example, using FMEA methodology to study the intensive insulin therapy process to achieve tight glucose control in the ICU would identify potential barriers and failures preventing successful implementation.
- A significant risk encountered in achieving tight glucose control in the 80–110 includes hypoglycemia.
-
Common pitfalls of insulin administration include administration and calculation errors that can result in 10-fold differences in doses of insulin.
- Other administration details, such as the type of IV tubing used and how the IV tubing is primed.
- Additionally, it can significantly affect the amount of insulin delivered to the patient and, thus, the glucose levels.
- If an inadequate amount of solution is flushed through to prime the tubing, the patient may receive saline rather than insulin for a few hours, resulting in higher-than-expected glucose levels and titration of insulin to higher doses.
- The result would then be an unexpectedly low glucose several hours later.
- Once failure modes such as these are identified, a fail-safe system can be designed so that failures are less likely to occur.
- The advantages of FMEA include its focus on system design rather than on a single incident, such as in Root Cause Analysis (RCA).
- By focusing on systems and processes, the learning and changes implemented will likely impact a more significant number of patients.
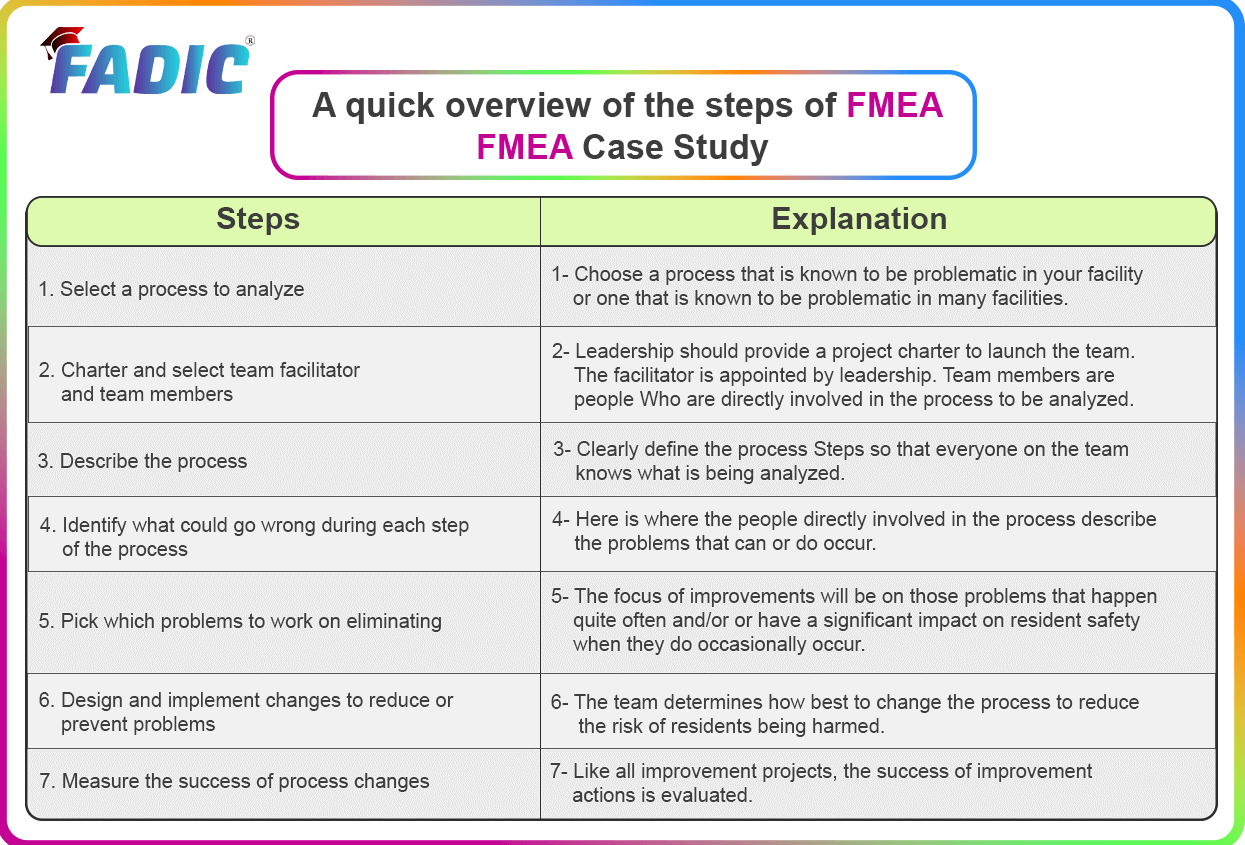
A quick overview of the steps of FMEA
- FMEA Case Study
Objective
- Administering medication to hospitalised infants and children is a complex process at high risk of error.
- Failure mode and effect analysis (FMEA) is a proactive tool used to analyse risks, identify failures before they happen and prioritise remedial measures.
- To examine the hazards associated with drug delivery to children, we performed a proactive risk-assessment analysis.
Design and setting
- Five multidisciplinary teams represent different divisions of the paediatric department at Padua University Hospital.
- However, it was trained to analyse the drug-delivery process.
- Additionally, to identify possible causes of failures and their potential effects, calculate a risk priority number (RPN) for each loss and plan practice changes.
Primary outcome
- To identify higher-priority potential failure modes as defined by RPNs and plan changes in clinical practice to reduce the risk of patient harm and improve safety in medication use in children.
Results
- In all, 37 higher-priority potential failure modes and 71 associated causes and effects were identified. The highest RPNs related (>48) mainly to errors in calculating drug doses and concentrations.
- Many of these failure modes were found in all five units, suggesting the presence of common targets for improvement, particularly in enhancing the safety of prescription and preparation of endovenous drugs.
- The introduction of new activities in the revised drug administration process reduced the high-risk failure modes by 60%.
Conclusions
- FMEA is an effective proactive risk-assessment helpful tool to aid multidisciplinary groups in understanding process care and identifying errors that may occur, prioritising remedial interventions and possibly enhancing the safety of drug delivery in children.
FMEA vs RCA comparison

- Failure Mode and Effects Analysis (FMEA) is a structured way to identify and address potential problems.
- Additionally, identify the failures and their effects on the system or process before an adverse event occurs.
- In comparison, Root Cause Analysis (RCA) is a structured way to address problems after they occur.
Conclusion
- Failure Modes and Effects Analysis (FMEA) differs from Root Cause Analysis (RCA).
- Root Cause Analysis (RCA) is a reactive process employed to identify its underlying causes after an error occurs.
- Additionally, FMEA is a proactive process used to look more carefully and systematically at vulnerable areas or functions.
Read More:
- Antimicrobial Stewardship School
- Sepsis Training Program
- Download Pocket Guide for Antibiotic Pharmacotherapy Book
- Microbiology Course | ABC Bacteria
- Infectious Disease E-News | FREE Subscription
- ABC antimicrobials | Know all about the Antimicrobials
- Road Map to Antimicrobial Stewardship Training Program
- Register Now at FADIC Clinical Research School
- FADIC Drug Information Fellowship
- Buy FADIC Toolkit for Writing Research to Write a Great Research Paper
- Read 10 Skills You Must Learn to Do Research via Google Scholar in Arabic
- The FADIC Online Continuous Medical Improvement Programs & Mini-Courses.
- Check Now the FADIC Book store and Buy books in different specialities.
- Watch Now FADIC TV to Keep Yourself Updated.
- FADIC Podcast focuses on varieties of pharmacist perspectives in different specialities.
- Subscribe Now to FADIC 2020 Daily News (FNN) and Keep Updated.
- Check Now about Coronavirus Resource Information Center.

 Log in
Log in Sign up
Sign up