Why you need need to learn this Course ?
It is important to Understand the content of the Clinical Trials, the Terminology. To know the phases of the Clinical Trials, and to learn the Top 10 clinical trials websites. It really deserves to learn how to understand and interpret the clinical trials
Overview
Interpretation of Clinical Trial Course
“How to Read and Understand the Clinical Trials?”
Learning Objectives:
- Describe the special characteristics of Clinical Trials and Understand it.
- Identify the top clinical trials websites, such as clinical trials gov.
- Discuss methods to identify the potential and conclusions of clinical trial.
- Differentiate between internal and external validity of clinical research.
- Identify key information presented in sections of a clinical trial and
phases of clinical trials.
- Discuss the need for well-defined study objectives, randomisation, and blinding within a clinical trial.
- Understand the clinical trial phases.
| What You Will Get Every Week (Lesson Planning) | |||
| Online Lesson | Workshop / Project | Mentor Support | Reviewer |
| (2-3 Hours) per week | (60 Minutes) per week | Every week |
Review the answers with feedback
|
Introduction Session:
Lecturer:
Dr. Rasha Abdelsalam
BCPS - AQ (ID), CPHQ, TQM (AUC),
Master & Diploma of Clinical Pharmacy.
Quantitative Research Certification.
Antimicrobial Stewardship Certification/SIDP.
Item Writing American Board of Infectious Disease.
Reviewer for PSAP ID 2018 Series

Dr. Rasha is a CEO of FADIC. She was in Tanta university hospital, Egypt. In 2013, she received TQM from American university, BCPS, CPHQ, and M.Sc. of Clinical Pharmacy. She received certification from SIDP. She obtained the add qualification from American board in infectious disease. Leaded MM CBAHI Saudi accreditation. Established stewardship committee, and implementation in MCH, Makkah, Saudi Arabia.
She shared in item exam writing for BCIDP Exam. She shared as a reviewer in infectious diseases PSAP. Authorship for published around 20 papers. One paper about vision of 2030 of general pharmaceutical department in Saudi Arabia. She received TLC certification from ACCP academy. Moreover, consultant on personal, professional, stewardship leadership, research. Share in strategic planning; hospital accreditation; and stewardship education.
Related Mini-Courses:
- FADIC Drug Information Fellowship Course
- Drug Information Program and Workshop
- Biostatistics Clinical Guide
- Medication Errors: How to Avoid?
- Systematic Review Course
- Journal Club Presentations Course
- Applied Medical Resources
- FADIC Pharmacokinetics Program
- The Future Guide for Pharmacist
Related Books:
- Clinical Practice Guidelines Tools & Resources E- Book
- Pharmacy Regulations Book
- FADIC Clinical Pharmacology Cards Quick Guide
Keep Learning:
- FADIC® – Online Programs & Mini-Courses
- FADIC Articles (English & Arabic) Blog
- FADIC® E-Book Store
-
Week 1
- Introduction of Clinical Trial
- Clinical Trial and Literature Evaluation
- Content of Clinical Trials
- Experimental Study Design
- Workshop of Clinical Trial
-
Week 2
- Internal & External Validity
- Biases and Confounding
- Incidence & Prevalence
- Top 10 Clinical Trial Websites
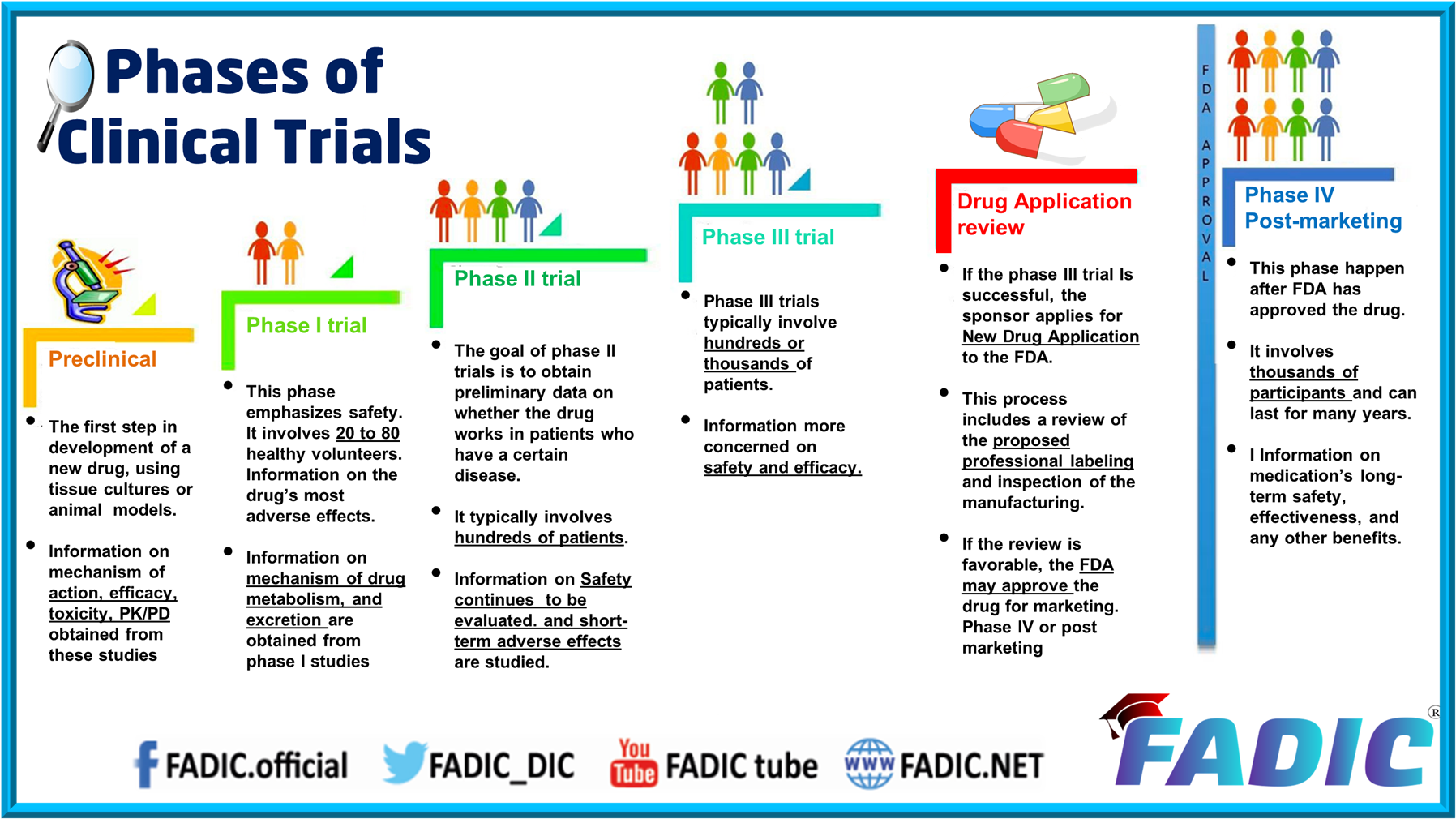
- Phases of Clinical Trials
- Phase 4 and Conclusion
- Final Project of Clinical Trial
-
Your Certificate
- And Finally, Your Certificate…

 Log in
Log in Sign up
Sign up