Coronavirus Experimental Treatment
Coronavirus Resource Information Center
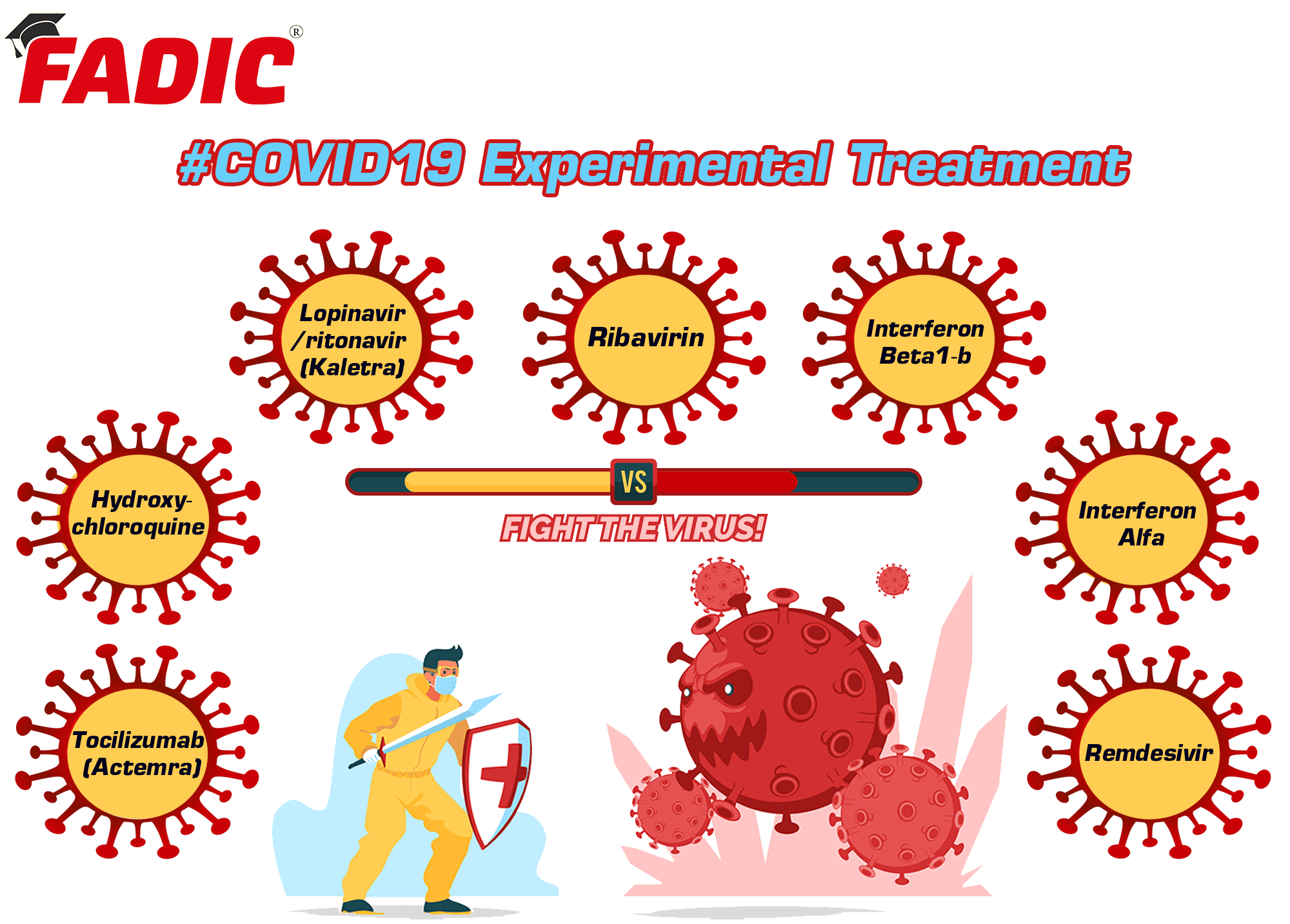
- Tocilizumab (Actemra)
- Hydroxychloroquine
- Lopinavir/ritonavir (Kaletra)
- Ribavirin
- Interferon Beta-1b
- Interferon Alfa
- Remdesivir
By: Rasha Abdelsalam
Introduction of Coronavirus Resource
- Coronavirus disease (COVID-19) is an infectious disease caused by a newly discovered coronavirus.
- In addition to most people infected with the COVID-19 virus will experience mild to moderate respiratory illness and recover without requiring special treatment.
- Moreover, older people, and those with underlying medical problems like cardiovascular disease, diabetes. In addition to chronic respiratory disease.
- Besides, the cancer are more likely to develop serious illness.
- However, the best way to prevent and slow down transmission is be well informed about the COVID-19 virus, the disease it causes and how it spreads.
- Then, the COVID-19 virus spreads primarily through droplets of saliva or discharge from the nose when an infected person coughs or sneezes.
- In addition to, protect yourself and others from infection by washing your hands or using an alcohol based rub frequently and not touching your face.
- At this time, there are no specific vaccines or treatments for COVID-19.
- However, there are many ongoing clinical trials evaluating potential treatments.
COVID-19 Experimental Treatment in the Coronavirus Resource Centre
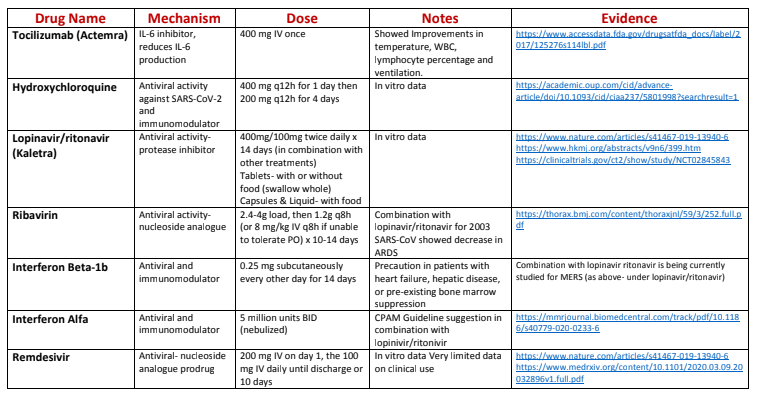
Tocilizumab (Actemra)
Mechanism of Action:
- IL-6 inhibitor, reduces IL-6 production which is a key cytokine produced in COVID-19
Best Candidates/Precautions:
- Patients with severe/critical illness with excessive inflammatory response
Dose:
- 400 mg IV once
Data to support:
- Moreover, 21 patients with severe/critical illness.
- In addition to, improvements in temperature, WBC, lymphocyte percentage and ventilation (from this LINK)
Hydroxychloroquine
Mechanism of Action:
- Antiviral activity against SARS-CoV-2 and immunomodulator
Best Candidates/Precautions:
- Precaution in patients with cardiac disease or other risk for prolonged QTc.
Dose:
- 400 mg q12h for 1 day then 200 mg q12h for 4 days
Data to support:
- Importantly, in vitro data showing antiviral activity with hydroxychloroquine & PK modeling dosing, from this LINK.
Lopinavir/ritonavir (Kaletra)
Mechanism of Action:
- Antiviral activity- protease inhibitor
Best Candidates/Precautions:
- Appears to be best used in combination with other therapies.
- In addition to, precaution in patients with cardiac disease or other risk for prolonged QTc.
Dose:
- 400mg/100mg twice daily x 14 days (in combination with other treatments)
- Tablets- with or without food (swallow whole)
- In addition to, Capsules and Liquid- with food
Data to support:
- In vitro data shows better antiviral activity of remdesivir and IFN Beta-1b compared with lopinavir/ritonavir, from this LINK.
- Finally, data for other coronaviruses in combination with other treatments like ribavirin or IFN Beta-1b
Ribavirin
Mechanism of Action:
- Antiviral activity- nucleoside analogue
Best Candidates/Precautions:
- Appears to be better in combination with lopinavir/ritonavir.
- In addition to, the contraindicated in pregnancy (birth defects- including male mediated). Several precautions, including avoiding use in unstable cardiac disease.
Dose:
- 2.4-4g load, then 1.2g q8h (or 8 mg/kg IV q8h if unable to tolerate PO) x 10-14 days
Data to support:
- In addition to, the combination with lopinavir/ritonavir for 2003 SARS-CoV showed decrease in ARDS or death as compared with ribavirin alone, from this LINK.
Interferon Beta-1b
Mechanism of Action:
- Antiviral and immunomodulator
Best Candidates/Precautions:
- In addition to, precaution in patients with heart failure, hepatic disease, or pre-existing bone marrow suppression
Dose:
- 0.25 mg subcutaneously every other day for 14 days
- In addition to, the data to support:Combination with lopinavir ritonavir is being currently studied for MERS.
Interferon Alfa
Mechanism of Action:
- Antiviral and immunomodulator
Best Candidates/Precautions:
- In addition to, potential for systemic absorption and reversible airflow obstruction, from this LINK.
Dose:
- 5 million units BID (nebulized)
Data to support:
In addition to, the CPAM Guideline suggestion in combination with lopinivir/ritonivir, from this LINK.
Remdesivir
Mechanism of Action:
- Antiviral- nucleoside analogue prodrug
Best Candidates/Precautions:
- Not currently FDA approved. In addition to availability through the manufacturer only.
Dose:
- 200 mg IV on day 1, the 100 mg IV daily until discharge or 10 days
Data to support:
- In vitro data shows antiviral activity, from this LINK.
- In addition to, very limited data on clinical use (pre-print, not peer reviewed), from this LINK.
Download Corona Virus Experimental Treatments in Coronavirus Resource Centre File from FADIC, from this LINK.
Reading Materials:
- Learn More about COVID-19 info-graph, from this LINK.
- In addition to, the important Role of Pharmacist info-graph, from this LINK.
- Download Now info-graphs of Role of Pharmacist in COVID-19, from this LINK.
Read More:
- Subscribe Now in FADIC 2020 COVID19 Daily News (FNN) and Keep Updated.
- In addition to, check Now about Coronavirus Resource Information Center.
- Learn More about all What you need about Cornonavirus
- Read More about COVID 19 Patient Education from FADIC
- In addition to, Corona Virus Experimental Treatment from FADIC
- All What you need about Cornonavirus from Arabic Blog
- Learn More about Cornonavirus from Arabic Blog
- In addition to, read more about Antimicrobial Stewardship Strategies.

 Log in
Log in Sign up
Sign up