Why FADIC Pharmacogenomic Program ?
To improve patient outcomes by maximizing efficacy and minimizing the toxicity of drug therapy through understanding the basic concept of pharmacogenomics
Overview
Pharmacogenomics and Personalized Medicine Certification Program
📘 Pharmacogenomics Free Online Course and Training Program
- Pharmacists and healthcare professionals often search for a pharmacogenomics free online course to build knowledge in personalised medicine. The FADIC Pharmacogenomics Training Program offers free introductory sessions alongside a structured certification course. These online modules cover the fundamentals of pharmacogenetics, pharmacogenomics, and their clinical applications, making it accessible for students and professionals worldwide.
💡 Pharmacogenomics Course Online with Certification
- If you are looking for a pharmacogenomics course online or a pharmacogenomics certification, this program provides a complete learning pathway. Participants can enrol in a five-week training course that combines online lectures, workshops, mentorship, and projects. At the end of the program, learners receive a recognised pharmacogenomics certificate, which demonstrates their expertise in genetically guided drug therapy and personalised medicine.
🎯 Pharmacogenomics Courses for Pharmacists and Clinicians
- The demand for pharmacogenomics pharmacist certification has grown as precision medicine becomes central to healthcare practice. This program is tailored for pharmacists, medical professionals, clinical scientists, and laboratory specialists. The course integrates CPIC guidelines, FDA-labelled pharmacogenomic biomarkers, and real clinical case studies, offering practical knowledge that can be directly applied to patient care.
🌍 Why Choose a Pharmacogenomics Certification Program
- Completing a pharmacogenomics certificate not only strengthens your career prospects but also improves your ability to apply precision medicine in clinical settings. The program highlights drug–gene interactions, pharmacokinetics, pharmacodynamics, and cost-effectiveness models, preparing you to meet the growing need for pharmacogenomic services in hospitals, pharmacies, and research institutions. With mentorship, project-based learning, and long-term access to updated course material, this online training ensures you stay ahead in the evolving field of personalised medicine.
Welcome to FADIC Pharmacogenomics Training Certification Program!!
- The mission of this program is to improve patient outcomes by maximising efficacy and minimising the toxicity of drug therapy.
- Through understanding the basic concept of pharmacogenomics.
- After you enrol in this program, you can take pharmacogenomics free online course symposium from FADIC as well.
In addition to teaching and service focused on genetically-guided drug therapy decision-making, drug discovery, and drug development.
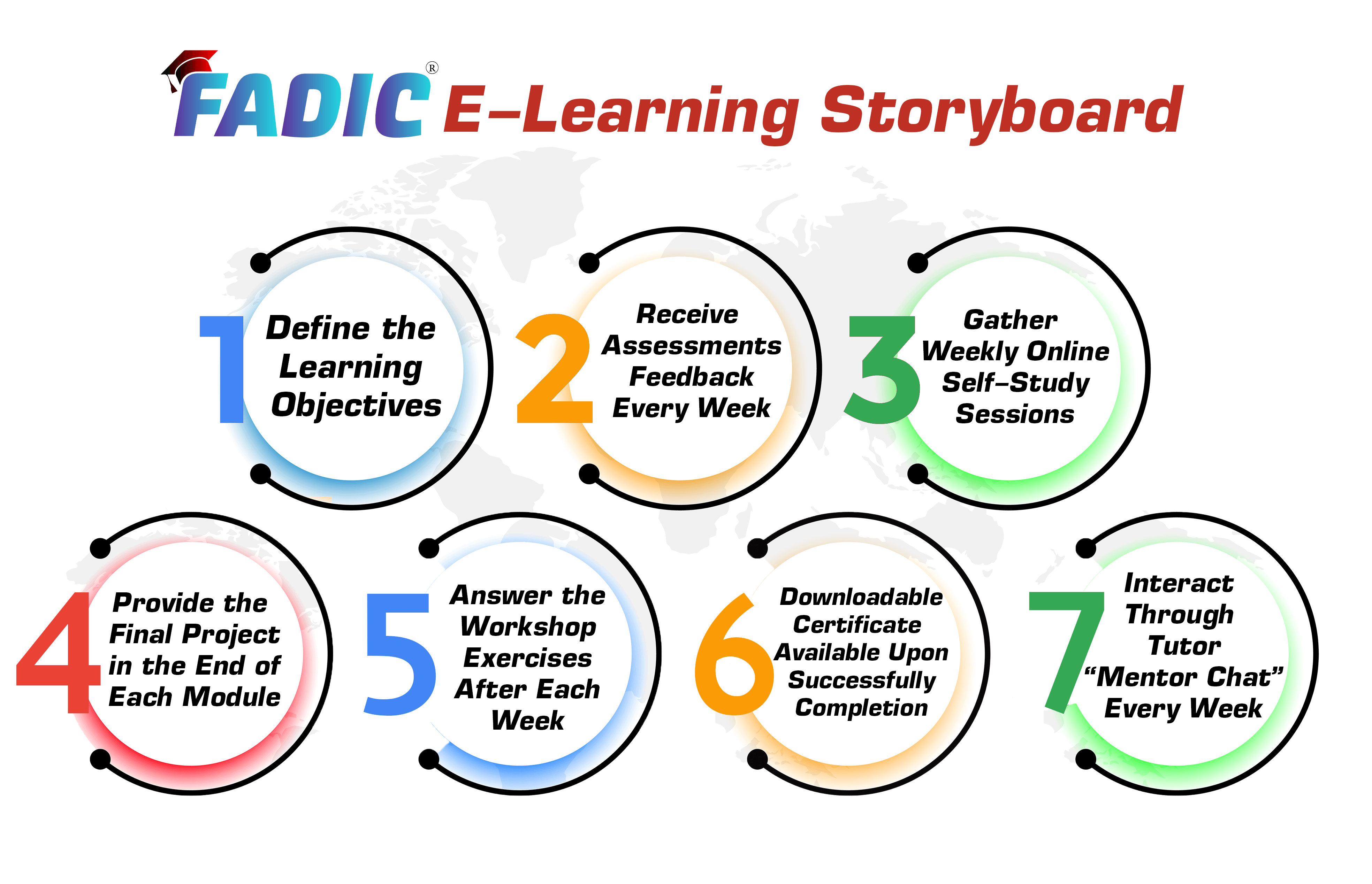
| What You Will Get Every Week (Lesson Planning) | |||
| Online Lesson | Workshop / Project | Mentor Support | Reviewer |
| (2-3 Hours) per week | (60 Minutes) per week | Every week | Review the answers with feedback |
What is Pharmacogenomics?
- Pharmacogenetics: the study of how genetic differences in a SINGLE gene influence variability in drug response (i.e efficacy and toxicity) by influencing the drug’s PK or PD.
- Pharmacogenomics: the study of how genetic (genome) differences in MULTIPLE genes influence variability in drug response (i.e efficacy and toxicity) by influencing the drug’s PK or PD
- Used interchangeably. The preferred term is Pharmacogenomics
Objectives of Pharmacogenomics Training Certification Program
- Firstly, Train clinical and basic scientists in pharmacogenomics.
- Secondly, Educate the concepts of pharmacogenomics, including research findings, potential implications, and implementations.
- Thirdly, Announcement for the FADIC pharmacogenomics initiative that includes various educational initiatives such as seminar series, workshops, research, and symposia.
* Duration: 5 Weeks
* Pharmacogenomics Training Certification Program Education System
- 📍Online Sessions
- 📍Workshops| Projects
- 📍Mentorship System
- 📍Reviewer System
- 📍Webinars
📝 The following topics will be covered at the Pharmacogenomics Training Certification Program
1- Personalized Medicine and Introduction to Pharmacogenomic
- Definition of Pharmacogenomics/ Pharmacogenetics
- Additionally, genomic achievements since the Human Genome Project and launching of the Personalised Medicine Initiative (PMI) by President Obama in 2015.
2- All Pharmacogenomics Terminology
3- Clinical Implementation of Pharmacogenomics
The importance of Pharmacogenomics in enhancing the therapeutic decisions.
- Clinical relevance of pharmacogenomics.
4- Application of pharmacogenomics in therapeutics (by area)
- CPIC Guidelines
- Examples of drug-gene(s) interactions
- In addition to, pharmacogenomics biomarkers in FDA drug labels
5- The Business model in pharmacogenomics (cost-effectiveness and Pharmacogenomics)
- Examples of clinical implementation of Pharmacogenomics, e.g. IGNITE, UTHealth Personalized Medicine Program, FDA precision medicine, and Obama Initiatives.
- The Challenges in Pharmacogenomic testing
- Challenges in the clinical implementation of Pharmacogenomics.
6- Your Role in Pharmacogenomics/ personalized medicine.
- How to increase awareness on pharmacogenomics?
- Additionally, recognize useful web tools/ resources/ databases to be able to gather information and answer questions about specific drug-gene(s) interactions.
- Moreover, journal Club Presentations: Pharmacogenomics-related paper to be selected, presented and discussed.
- Finally, the final project of the pharmacogenomics program.
The pharmacogenomics free online course symposium will offer also many examples of clinical application of pharmacogenomics.
Target Participants:
- All Pharmacy Specialities.
- All Medical Specialities.
- Clinical Laboratories, Consultancy, Pharmaceutical Services, and other Healthcare Professionals interested in pharmacogenomics.
Importance of Pharmacogenomics Program
Enhance the quality of pharmacogenomic research, and increasing multidisciplinary collaboration among these faculties. Specific areas of pharmacogenomics initiatives include:
- Firstly, The genetic basis of variability in drug efficacy and toxicity, including the study of genetic polymorphisms in drug targets. In addition to the drug transporters, and drug metabolizing enzymes.
- Secondly, This initiative will include the study of the genetic influences on drug pharmacokinetics and pharmacodynamics and will range from polymorphism discovery to in vitro or transgenic functional analysis of polymorphisms to small studies in humans of the functional effects of polymorphisms to large pharmacogenomic clinical trials.
- Finally, Investigation of disease-gene associations, especially those that may have relevance to pharmacogenomics. Use of human genetic information in drug discovery and development.
What is the Concept of Pharmacogenomics?
- The concept of management in the health sector and the pharmaceutical sector is to understand the broadest sense of the term and includes public sector services.
- Moreover, to understand the clinical centers and services belonging to health care institutions at any level of care. It also helps to understand the pharmaceutical service providers. In addition to, the agencies for the evaluation of drugs and medical technologies.
- Together, this will add to the pharmaceutical industry, pharmaceutical distributors, insurance companies. In addition to the other consultancy and advisory firms.
Importance of Learning Pharmacogenomics?
- The Online Program of Pharmacoeconomics is of special relevance for professionals, pharmacists, and other graduates of the health sector.
- The program address all the issues of health pharmacoeconomics. Moreover, it targets the design and management of quality programmes.
- The programme targets all graduates and health sector professionals. In addition to, it offers an academic training with a high reputation on the market. Participants are not required to have previous knowledge of pharmacogenomics.
Who is it for Pharmacogenomics Training Certification Program?
- This program aims at professionals in the health sector.
- In addition to the pharmaceutical sector who cannot afford the time to attend an on-site course.
- Moreover, the program aims those who prefer to work primarily through virtual communication.
- This International program in Pharmacogenomics meets the needs and requirements both of experienced professionals.
- In addition to the other recent graduates who wish to increase their knowledge of these areas.
- The programme addresses all professionals who, wherever in the world.
- It addresses those who live and wishes to follow a distance program with the characteristics we have outlined above.
- It is conducted entirely online.
- In particular, the programme designs to complement the training of professionals with management responsibilities.
- Additionally, the graduates interested in acquiring the necessary skills to take on such responsibilities in:
- Hospitals, clinical laboratories and public and private health centres.
- Public bodies responsible for managing and/or funding pharmaceuticals and health services.
- Private insurance companies.
- Pharmaceutical companies and companies related to the distribution of pharmaceuticals.
- Pharmaceutical and health service management training centres.
- Consultancy firms related to the health sector, etc.
- Specialist Doctors, Consultants. In addition to other healthcare professionals.
With FADIC Pharmacogenomics Training Certification Program, you Will Have…
Enhance your learning with the FADIC Mentorship Reviewer System. A dedicated mentor will guide you through your projects and workshops, providing personalized feedback to help you succeed.
Maximize your learning with downloadable and printable FADIC handouts. Study effectively and independently at your own pace.
Stay Ahead with FADIC: 24-Month Access to All Updates and Course Materials
Stay updated with FADIC: Starting from your registration date, you will enjoy 24 months of full access to all course materials, including the latest updates and course content.

Pharmacogenomics Free Online Course Symposium
If you have any inquiry, please contact WhatsApp.

Week 1
- Introduction Session of FADIC Pharmacogenomic Program
- Pharmacogenomics and Personalized Medicine
- Historical Perspective and Genomic Achievements
- Personalized Medicine and Precision Medicine Initiative
- Workshop 1 of Pharmacogenomic Program
Week 2
- Motivation for Learning Pharmacogenomics
- Basic Principles of Population Pharmacogenomics and its Application
- Example of Allele and Genotype Frequencies
- Examples of Human Drug – Gene(s) Interactions
- Pharmacogenomics Terms and Glossary
- Workshop 2 of Pharmacogenomic Program
Week 3
- Implementation of Pharmacogenomics into Clinical Practice
- Examples of Genetic Variants
- FDA Pharmacogenomic Bio-markers in Drug Labeling
- Workshop 3 of Pharmacogenomic Program
Week 4
- Integration of Pharmacogenomics information in FDA drug
- Challenges to Clinical Implementation of Pharmacogenomics
- Barriers to Clinical Implementation of Pharmacogenomics
- Examples of Pharmacogenomics Educational Initiative
- Workshop 4 of Pharmacogenomic Program
Pharmacogenomic Webinar
- Useful Pharmacogenomics Web Resources / Databases
- Clinical Pharmacogenomics (English)
- Webinar: The Pharmacist’s Role in Clinical Pharmacogenomics
Final Project
- Project: Example of the Drug-Gene Interaction Presentation
Your Certificate
- And Finally, Your Certificate…

 Log in
Log in Sign up
Sign up