Overview
Overview
Phases of Clinical Trials Workshop
What are clinical trial phases?
- Clinical trials are divided into different stages, called phases. The earliest phase of trials may determine whether a drug is safe or the side effects it causes.
What are trial phases?
- There are three main phases of clinical trials – phases 1 to 3. But some trials have an earlier stage called phase 0, and some phase 4 trials are done after a drug has been licensed.
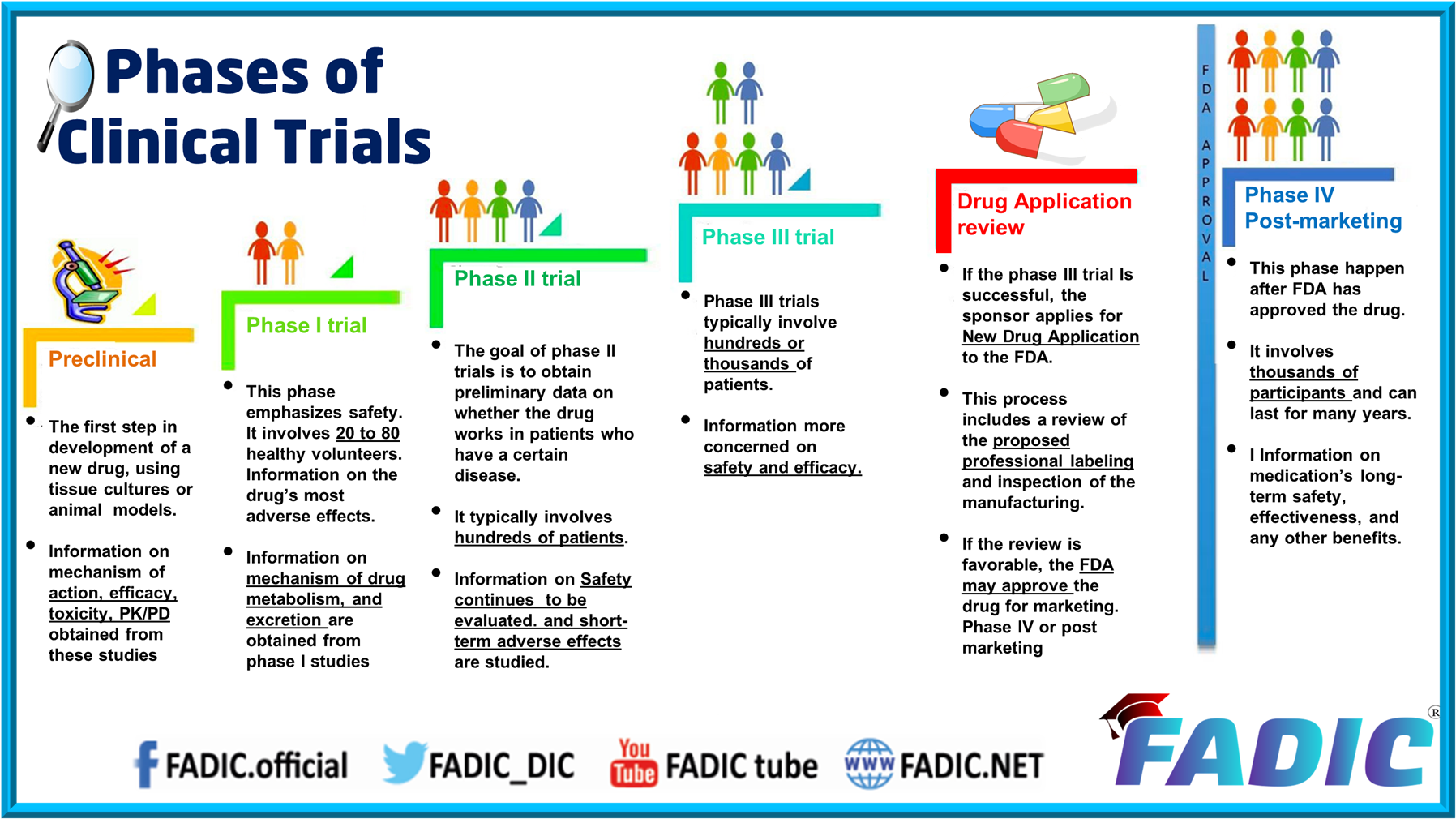
Pre-Clinical Phase
- Before pharmaceutical companies start clinical trials on a drug, they conduct extensive pre-clinical studies.
- These involve in vitro (test tube or cell culture) and in vivo (animal) experiments using wide-ranging doses of the study drug to obtain preliminary efficacy, toxicity and pharmacokinetic information.
- Such tests assist pharmaceutical companies in deciding whether a drug candidate has developed as an investigational new drug.
Phase 0
- Phase 0 trials are known as human micro-dosing studies designed to speed up the development of promising drugs.
- Phase 0 trials include administration of single sub-therapeutic doses of the study drug to a small number of subjects (10 to 15) to gather preliminary data on the agent’s pharmacokinetics (what the body does to drugs).
- A Phase 0 study gives no data on safety or efficacy.
Phase 1
- Phase I trials were formerly referred to as “first-in-man studies” Normally, a small group of 2–100 healthy volunteers will be recruited.
- These trials are often conducted in a clinical trial clinic, where the subject can be observed by full-time staff.
Phase 2
- Go the next goal is to evaluate whether the drug has any biological activity or effect.
- Phase II trials are performed on larger groups (100–300) and are designed to assess how well the drug works. Also, to continue Phase I safety assessments in a larger group of volunteers and patients.
Phase 3
- Phase III studies are randomised, controlled multi-centre trials on large patient groups (300–3,000 o more). They are the definitive assessment of how effective the drug is compared with the current ‘gold standard treatment.
- Phase III trials are the most expensive, time-consuming, and challenging to design and run, especially in therapies for chronic medical conditions.
Phase 4 and Post-marketing Surveillance
- A Phase IV trial is also known as a post-marketing surveillance trial or informally as a confirmatory trial. Phase IV trials involve safety surveillance (pharmacovigilance)
- The safety surveillance is designed to detect rare or long-term adverse effects over a much larger patient population and more extended time than was possible during the Phase I-III clinical trials.

Curriculum
-
Phases of Clinical Trials
- Content of Clinical Trials
- Phases of Clinical Trials
- Final Project
-
Your Certificate
- And Finally, Your Certificate…
Features
80 USD
Enroll Now
 Log in
Log in Sign up
Sign up