Creatinine Clearance Calculator
Creatinine Clearance Calculator
Content:
- Definition of Acute Kidney Injury
- Epidemiology of AKI
- Risk Factors of AKI
- Comorbid Conditions and Creatinine Clearance
- Drugs Associated with AKI
- Classification of Causes of AKI and Creatinine Clearance.
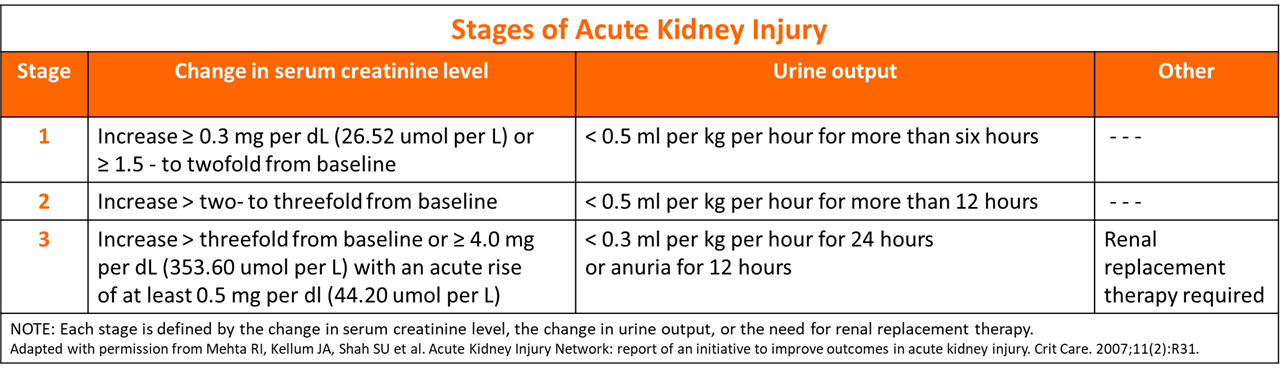
- Stages of AKI
- Diagnosis of AKI and Creatinine Clearance.
- Serum Creatinine Level and Creatinine Clearance.
- Pharmacological treatment of AKI
- Fluid resuscitation in AKI and Creatinine Clearance.
- Medications affected the AKI and Creatinine Clearance.
- Definition of Creatinine Clearance & formula
- Indications/applications
- Considerations in Creatinine Clearance
- Equations used in Creatinine Clearance
- The Cockcroft-Gault formula
- Drug Dosing According to Creatinine Clearance Calculation
- The MDRD Equation
- The CKD-EPI Equation
Definition of Acute Kidney Injury
- Acute kidney injury (AKI) refers to an abrupt decrease in kidney function.
- This is resulting in the retention of urea and other nitrogenous waste products and the dysregulation of extracellular volume and electrolytes.
- The term AKI has largely replaced acute renal failure (ARF), reflecting the recognition that smaller decrements in kidney function that do not result in overt organ failure are of substantial clinical relevance.
- Additionally, associated with increased morbidity and mortality.
- Several consensus definitions of AKI developed to provide a uniform definition of AKI.
- Moreover, these definitions based exclusively upon the serum Creatinine Clearance and urine output and are used primarily to identify patients with AKI in epidemiologic and outcome studies.
- Furthermore, the overall utility of these definitions and staging systems in the clinical assessment and management of patients with AKI remains to be validated.
Highly recommend online Marketing tool from SEMRUSH
Epidemiology of AKI
- The estimated incidence of AKI in cardiac surgery patients is 11–30%.
- Severe AKI requiring RRT occurs in an estimated 1–2% of these patients.
- In addition, numerous studies derived clinical risk scores of predictors of AKI after cardiac surgery.
- The strongest predictors of postoperative AKI among clinical risk scores are baseline kidney function and CKD status according to the Creatinine Clearance.
- Finally, the patients with decreases in serum creatinine levels from baseline immediately after surgery were less likely to develop AKI.
- All this compared to those whose serum creatinine levels increased after surgery.
Risk Factors of AKI
- age
- comorbid diseases
- proteinuria
- nephrotoxic exposures
- major surgery
- sepsis
- fluid resuscitation
- volume status.
- Older age increases the risk of AKI.
Comorbid Conditions and Creatinine Clearance
- Comorbid conditions including CKD, diabetes, hypertension, coronary artery disease, heart failure, liver disease, and chronic obstructive pulmonary disease are risk factors for AKI.
- In addition to the proteinuria with a GFR more significant than 60 mL/ minute/1.73 m2 or an elevated urinary albumin/creatinine
- Moreover, hospitalised patients, especially critically ill patients, exposed to several nephrotoxins and contrast exposure.
- Antimicrobials, NSAIDs, and proton pump inhibitors are common medications administered in this population.
- Acute kidney injury is common after cardiac surgery and is less common in the non-cardiac surgery population.
- Sepsis is a common predisposing factor to AKI, and the development of AKI further increases the risk of mortality.
- Choice of fluid for resuscitation may be a risk factor for AKI because hydroxyethyl starch increases the AKI risk compared with crystalloids.
- High-volume resuscitation with crystalloids has a higher risk of AKI than balanced salt solutions because of the harmful effects of chloride loading.
- Fluid overload and therapies to treat volume overload increase the risk of AKI and affect Creatinine Clearance.
Drugs Associated with AKI
Pre-renal
- ACEIs/ARBs
- Calcineurin inhibitors
- COX-2 inhibitors
- Diuretics
- NSAIDs
Glomerular Injury
- Interferon
- Pamidronate
Acute Interstitial Nephritis
- Allopurinol
- Azathioprine
- Chinese herbs – Stephania tetrandra, Magnolia officinalis.
- Cimetidine
- Diuretics (thiazides, furosemide)
- NSAIDs
- Phenytoin
- Proton pump inhibitors
- Quinolones
- Rifampin
- Semisynthetic penicillins (ampicillin, nafcillin, oxacillin)
- Sulfonamides
- Vancomycin
Acute Tubular Necrosis
- Aminoglycosides
- Amphotericin B
- Carboplatin
- Cisplatin
- Cyclophosphamide
- Ifosfamide
- Pentamidine
- Radiocontrast media
- Vancomycin
Crystal Nephropathy
- Acyclovir
- Allopurinol
- Indinavir
- Methotrexate
- Nelfinavir
- Quinolones
- Sulfonamides
- Triamterene
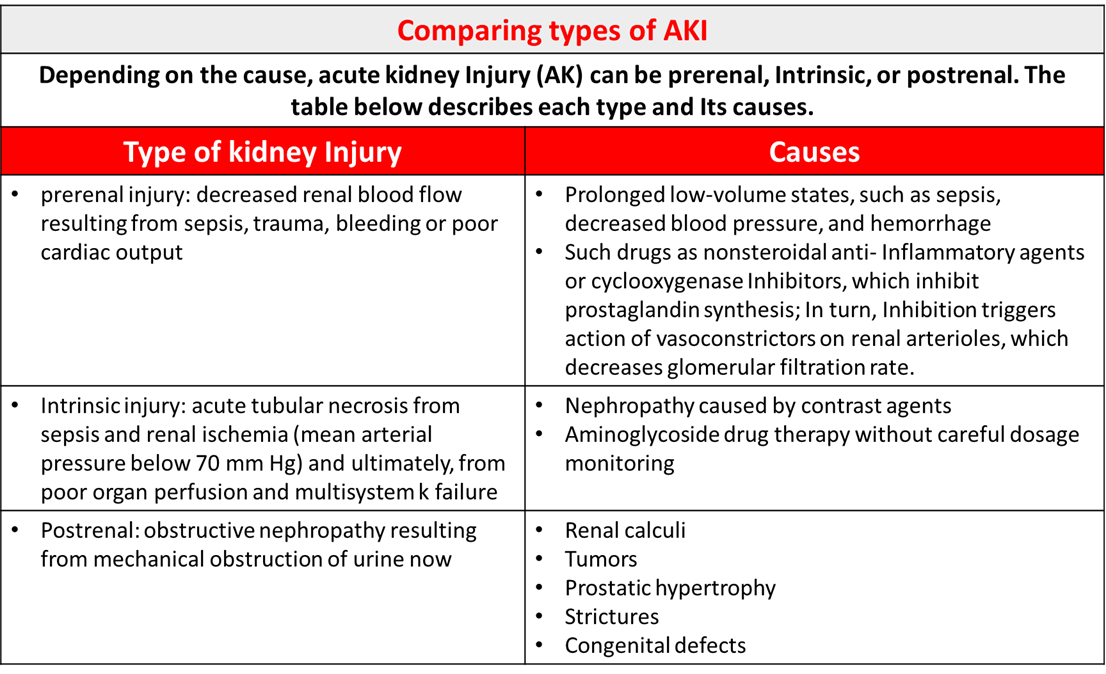
Classification of Causes of AKI and Creatinine Clearance.
Pre-renal
- Intrarenal vasoconstriction (hemodynamically mediated)
- Medications: nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, cyclosporine (Sandimmune), tacrolimus (Prograf)
- Cardiorenal syndrome
- Hepatorenal syndrome
- Abdominal compartment syndrome
- Hypercalcemia
- Systemic vasodilation (e.g., sepsis, neurogenic shock)
- Volume depletion
- Renal loss from diuretic overuse, osmotic diuresis (e.g., diabetic ketoacidosis)
- Extra-renal loss from vomiting, diarrhoea, burns, sweating, blood loss
Intrinsic renal (Interstitial)
- Medications: penicillin analogues, cephalosporins, sulfonamides, ciprofloxacin (Cipro), acyclovir (Zovirax), rifampin, phenytoin (Dilantin), interferon, proton pump inhibitors, nonsteroidal anti-inflammatory drugs
- Moreover, the infections (e.g., direct infection of renal parenchyma or associated with systemic infections)
- Viruses: Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus
- Bacteria: Streptococcus species, Legionella species
- Fungi: candidiasis, histoplasmosis
- Systemic disease: sarcoidosis, lupus
Intrinsic renal (Tubular)
- Ischemic: prolonged hypotension
- Nephrotoxic: exogenous toxins (e.g., radiographic contrast agents, aminoglycosides, cisplatin, methotrexate, ethylene glycol, amphotericin B).
- Additionally, the endogenous toxins (e.g., hemolysis and rhabdomyolysis [pigment nephropathy], tumour lysis syndrome, myeloma)
Intrinsic renal (Vascular)
- Renal vein thrombosis, malignant hypertension, scleroderma renal crisis, renal atheroembolic disease, and renal infarction
Intrinsic renal (Post-renal)
- Extra-renal obstruction: prostate hypertrophy; neurogenic bladder; retroperitoneal fibrosis; bladder, prostate, or cervical cancer.
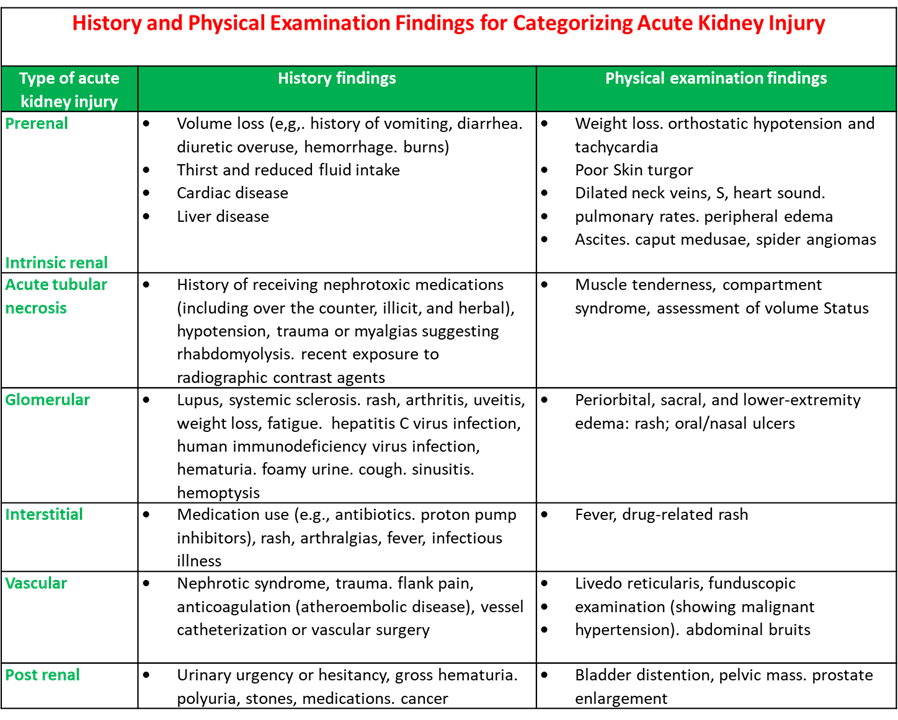
History and Physical Examination Findings for Categorising Acute Kidney Injury
Comparing Types of AKI
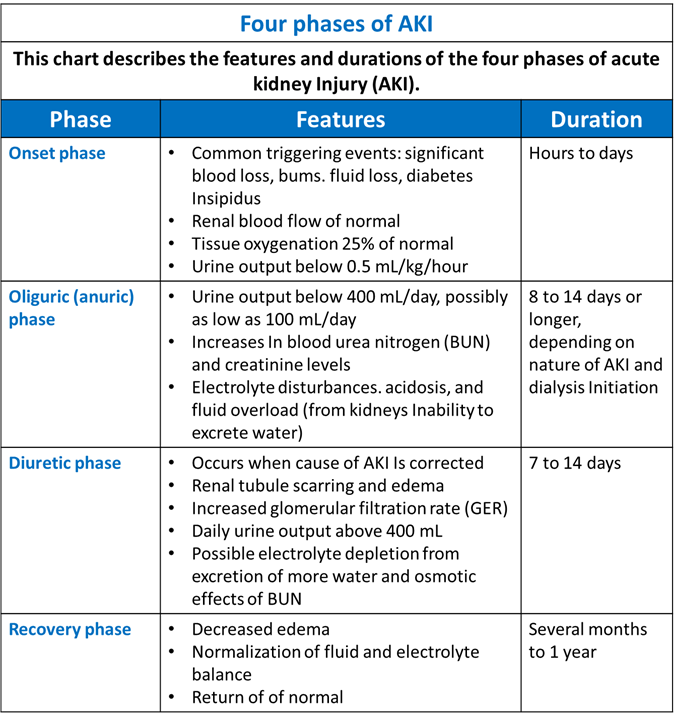
Stages of AKI
- Firstly, the Onset phase: Kidney injury occurs.
- Secondly, the Oliguric (anuric) phase: Urine output decreases from renal tubule damage.
- Thirdly, the Diuretic phase: The kidneys try to heal and urine output increases, but tubule scarring and damage occur.
- Finally, the Recovery phase: Tubular oedema resolves, and renal function improves. (See Four phases of AKI).
Four Phases of AKI
Stages of Acute Kidney Injury
Diagnosis of AKI and Creatinine Clearance.
- A patient history and physical examination, with an emphasis on assessing the patient’s volume status, are crucial for determining the cause of acute kidney injury.
- The history should identify the use of nephrotoxic medications or systemic illnesses that might cause poor renal perfusion or directly impair renal function.
- Physical examination should assess intravascular volume status and any skin rashes indicative of systemic illness.
- The initial laboratory evaluation should include urinalysis, complete blood count, and measurement of serum creatinine level and fractional excretion of sodium (FENa).
- Moreover, imaging studies can help rule out obstruction.
Serum Creatinine Level and Creatinine Clearance.
- It is essential to compare the patient’s current serum creatinine level with previous levels to determine the duration and understanding of the disease.
- The definition of acute kidney injury indicates that a rise in creatinine had occurred within 48 hours. However, it may be hard to ascertain when the rise happened in the outpatient setting.
- Additionally, the high serum creatinine level in a patient with a previously normal documented level suggests an acute process.
- Whereas a rise over weeks to months represents a subacute or chronic process.
Urine Analysis
- Urinalysis is the most critical noninvasive test in the initial workup of acute kidney injury.
- Moreover, the findings on urinalysis guide the differential diagnosis and direct further workup.
Complete Blood Work
- The presence of acute hemolytic anaemia with the peripheral smear showing schistocytes in the setting of acute kidney injury.
- It should raise the possibility of hemolytic uremic syndrome or thrombotic thrombocytopenic purpura.
Urine Electrolytes
- In patients with oliguria, measurement of FENa helps distinguish prerenal from intrinsic renal causes of acute kidney injury.
-
FENa is defined by the following formula:

- A value less than 1 percent indicates a prerenal cause of acute kidney injury, whereas a value greater than 2 percent indicates an intrinsic renal cause.
- In patients on diuretic therapy, however, a FENa higher than 1 percent may be caused by natriuresis induced by the diuretic and is a less reliable measure of a prerenal state.
- In such cases, fractional excretion of urea may be helpful, with values less than 35 percent indicating a prerenal cause.
- FENa values less than 1 percent are not specific for prerenal causes of acute kidney injury because these values can occur in other conditions.
- Such as contrast nephropathy, rhabdomyolysis, acute glomerulonephritis, and urinary tract obstruction.
Imaging Studies in AKI and Creatinine Clearance
- Renal ultrasonography performed in most patients with acute kidney injury, particularly in older men, to rule out obstruction (i.e., a post-renal cause).
- The presence of post-void residual urine more significant than 100 mL (determined by a bladder scan or via urethral catheterisation if bladder scan is unavailable) suggests post-renal acute kidney injury. It requires renal ultrasonography to detect hydronephrosis or outlet obstruction.
- Moreover, to diagnose extra-renal causes of obstruction (e.g., pelvic tumors), other imaging modalities, such as computed tomography or magnetic resonance imaging, may be required.
Renal Biopsy
- The Renal biopsy reserved for patients in whom prerenal and post-renal causes of acute kidney injury have been excluded, and the cause of intrinsic renal injury is unclear.
- Moreover, Renal biopsy is essential when clinical assessment and laboratory investigations suggest a diagnosis.
- Additionally, it requires confirmation before disease-specific therapy. (e.g., immunosuppressive medications) is instituted.
- Renal biopsy performed urgently in patients with oliguria who have rapidly worsening acute kidney injury, hematuria, and red blood cell casts.
- Furthermore, in this setting, in addition to indicating a diagnosis that requires immunosuppressive therapy.
- Moreover, the biopsy may support the initiation of special therapies, such as plasmapheresis, if Good pasture syndrome is present.
Diagnostic Test Results and Diseases in Patients with Acute Kidney Injury
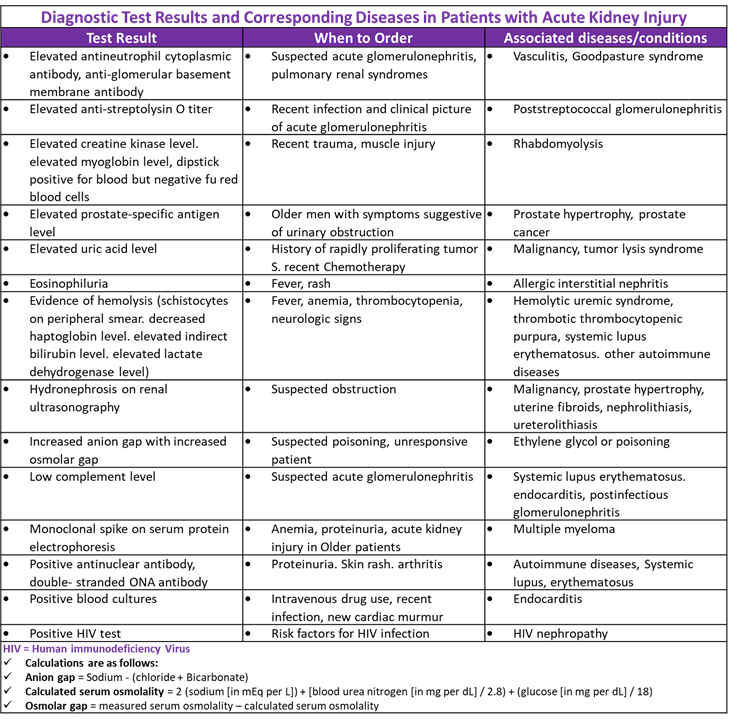
Pharmacological treatment of AKI
- Patients with acute kidney injury generally should be hospitalised unless the condition is mild and results from an easily reversible cause.
- The key to management is assuring adequate renal perfusion by achieving and maintaining hemodynamic stability and avoiding hypovolemia.
- In some patients, clinical assessment of intravascular volume status and avoidance of volume overload may be difficult.
- As in which case measurement of central venous pressures in an intensive care setting may be helpful.
Fluid resuscitation in AKI and Creatinine Clearance.
- If fluid resuscitation is required because of intravascular volume depletion, isotonic solutions (e.g., normal saline) are preferred over hyperoncotic solutions (e.g., dextrans, hydroxyethyl starch, albumin).
- A reasonable goal is a mean arterial pressure greater than 65 mm Hg, which may require the use of vasopressors in patients with persistent hypotension.
- Renal-dose dopamine is associated with poorer outcomes in patients with acute kidney injury; it is no longer recommended.
- Cardiac function optimised as needed with positive inotropes or afterload and preload reduction.
- Attention to electrolyte imbalances (e.g., hyperkalemia, hyperphosphatemia, hypermagnesemia, hyponatremia, hypernatremia, metabolic acidosis) is essential.
- Severe hyperkalemia defined as potassium levels of 6.5 mEq per L (6.5 mmol per L) or greater, or less than 6.5 mEq per L with electrocardiographic changes typical of hyperkalemia (e.g., tall, peaked T waves).
In severe hyperkalemia
- In severe hyperkalemia, 5 to 10 units of regular insulin and dextrose 50% intravenously can shift potassium out of circulation and into the cells.
- Calcium gluconate (10 mL of 10% solution infused intravenously over five minutes) is also used to stabilise the membrane and reduce the risk of arrhythmias when electrocardiographic changes show hyperkalemia.
- In patients without electrocardiographic evidence of hyperkalemia, calcium gluconate is not necessary.
- Still, sodium polystyrene sulfonate (Kayexalate) can be given to lower potassium levels gradually, and loop diuretics can be used in patients who are responsive to diuretics.
- Dietary intake of potassium should be restricted.
- The main indication for the use of diuretics is the management of volume overload.
- Intravenous loop diuretics can be helpful for this purpose as a bolus or continuous infusion.
- However, it is essential to note that diuretics do not improve morbidity, mortality, or renal outcomes.
- Additionally, not used to prevent or treat acute kidney injury without volume overload.
Medications affected the AKI and Creatinine Clearance.
- All medications that may potentially affect renal function by direct toxicity or hemodynamic mechanisms should be discontinued, if possible.
- For example, metformin (Glucophage) should not be given to patients with diabetes mellitus who develop acute kidney injury.
- The dosages of essential medications adjusted for the lower level of kidney function.
- Avoidance of iodinated contrast media and gadolinium is essential, and if imaging is needed, non-contrast studies are recommended.
- Supportive therapies (e.g., antibiotics, maintenance of adequate nutrition, mechanical ventilation, glycemic control, anaemia management)
- All these pursued based on standard management practices.
- In patients with rapidly progressive glomerulonephritis, treatment with pulse steroids, cytotoxic therapy, or a combination may be considered, often after confirmation of the diagnosis by kidney biopsy.
- In some patients, the metabolic consequences of acute kidney injury cannot be adequately controlled with conservative management, and renal replacement therapy will be required.
- The indications for initiation of renal replacement therapy include refractory hyperkalemia, volume overload refractory to medical management, uremic pericarditis or pleuritis, uremic encephalopathy, intractable acidosis, and certain poisonings and intoxications (e.g., ethylene glycol, lithium).
- There is no specific pharmacologic therapy proven to treat AKI secondary to hypoperfusion and/or sepsis.
- The only therapeutic or preventive intervention that established beneficial effects in managing AKI is the intravenous (IV) administration of isotonic sodium chloride solution.
- Furthermore, it should be given in quantities sufficient to keep the patient euvolemic or even hypervolemic.
Diuretics, Loop
- Although diuretics do not affect the outcome of established AKI, they appear helpful in fluid homeostasis and are used extensively.
- Moreover, they used to reduce the requirement for renal replacement therapy.
- The use of isotonic sodium chloride solution in conjunction with diuretics is debatable.
Furosemide (Lasix)
- Furosemide increases the excretion of water by interfering with the chloride-binding co-transport system, which, in turn, inhibits sodium and chloride reabsorption in the thick ascending loop of Henle and the distal renal tubule.
- Additionally, it is a potent and rapid-acting agent with peak action at 60 minutes and a 6- to the 8-hour duration of action.
- In renal failure, higher doses must be used for a more significant diuretic effect.
- Doses as high as 600 mg/day may be needed under monitored conditions.
- Frequently, IV doses are needed in AKI to maintain urine output.
- IV infusions are often helpful in intensive care settings where larger doses are necessary.
- In addition, this method promotes a sustained natriuresis with reduced ototoxicity compared with conventional intermittent bolus dosing.
Inotropic Agents
- Dopamine in small doses (e.g., 1-5 mcg/kg/min) causes selective dilatation of the renal vasculature, enhancing renal perfusion.
- Besides, Dopamine also reduces sodium absorption, thereby decreasing the energy requirement of the damaged tubules.
- This enhances urine flow, which, in turn, helps to prevent tubular cast obstruction.
- Furthermore, the clinical benefit of low-dose dopamine remains uncertain.
Dopamine
- Dopamine stimulates adrenergic and dopaminergic receptors.
- Its hemodynamic effect is dose-dependent.
- Moreover, the lower doses (0.5-3.0 mcg/kg/min) predominantly stimulate dopaminergic receptors, producing renal and mesenteric vasodilation.
- Higher doses produce cardiac stimulation and renal vasodilation.
- Moreover, Potential complications of dopamine use include cardiac arrhythmias, myocardial ischemia, and intestinal ischemia.
Vasodilators
- Fenoldopam decreases systemic vascular resistance and increases renal blood flow to the cortex and medullary regions in the kidney.
- Besides, noted to improve renal function in patients with severe hypertension.
Fenoldopam (Corlopam)
- Fenoldopam is a selective dopamine-receptor agonist that acts as a rapid-acting vasodilator.
- It is six times more potent than dopamine in producing renal vasodilation.
- Moreover, it increases diuresis and has minimal adrenergic effects.
- In addition, Fenoldopam is indicated for treating severe hypertension, including in patients with renal compromise.
Calcium Channel Blockers
- These drugs are effective in animal models of AKI, but their efficacy not proven in humans.
- The effects of calcium channel blockers are believed to be mediated through vasodilation and used to enhance the function of transplanted kidneys.
Nifedipine (Adalat, Procardia, Afeditab CR, Nifediac CC, Nifedical XL)
- Nifedipine relaxes smooth muscle and produces vasodilation, which, in turn, improves blood flow and oxygen delivery.
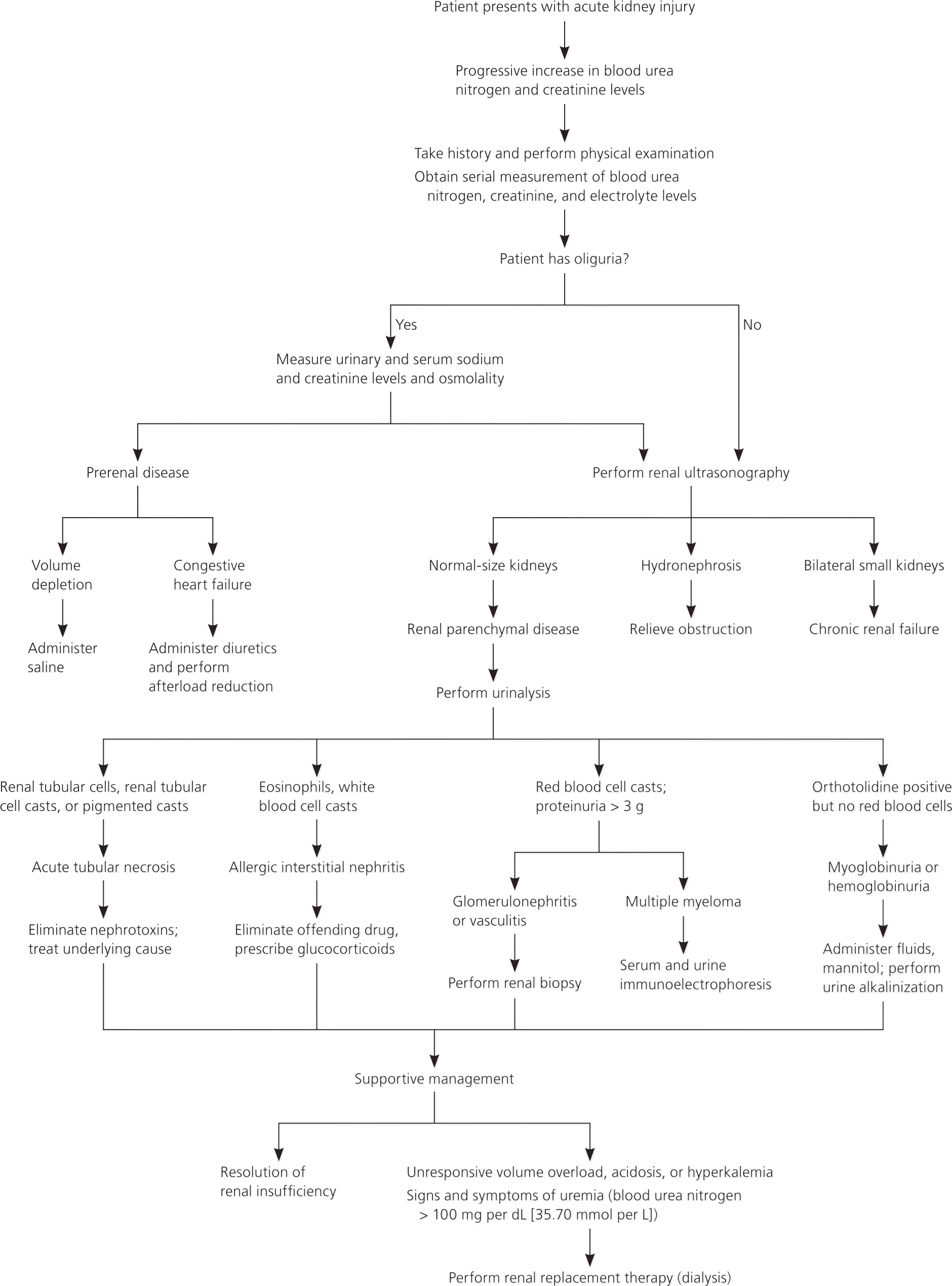
Creatinine Clearance
Definition of Creatinine Clearance.
- Creatinine is critically important in assessing renal function because it has several interesting properties.
- In blood, it is a marker of glomerular filtration rate; in urine, it can remove the need for 24-hour collections for many analytes or be used as a quality assurance tool to assess the accuracy of a 24-hour collection.
- Creatinine forms at a relatively constant rate in muscle.
- Chemically, it is the anhydride (dehydration product) of creatine; once formed, it cannot be converted back into creatine.
- Therefore, the amount of creatinine formed daily is related to muscle mass, which varies with ethnicity, age, and gender.
- Besides, Creatinine is freely filtered through the glomerulus and is not appreciably reabsorbed or secreted by the renal tubules.
- As a result, the serum concentration of creatinine represents a balance between its production (related to muscle mass) and the glomerular filtration rate (GFR).
- For any given patient, the serum creatinine is constant so long as muscle mass remains unchanging and GFR is constant.
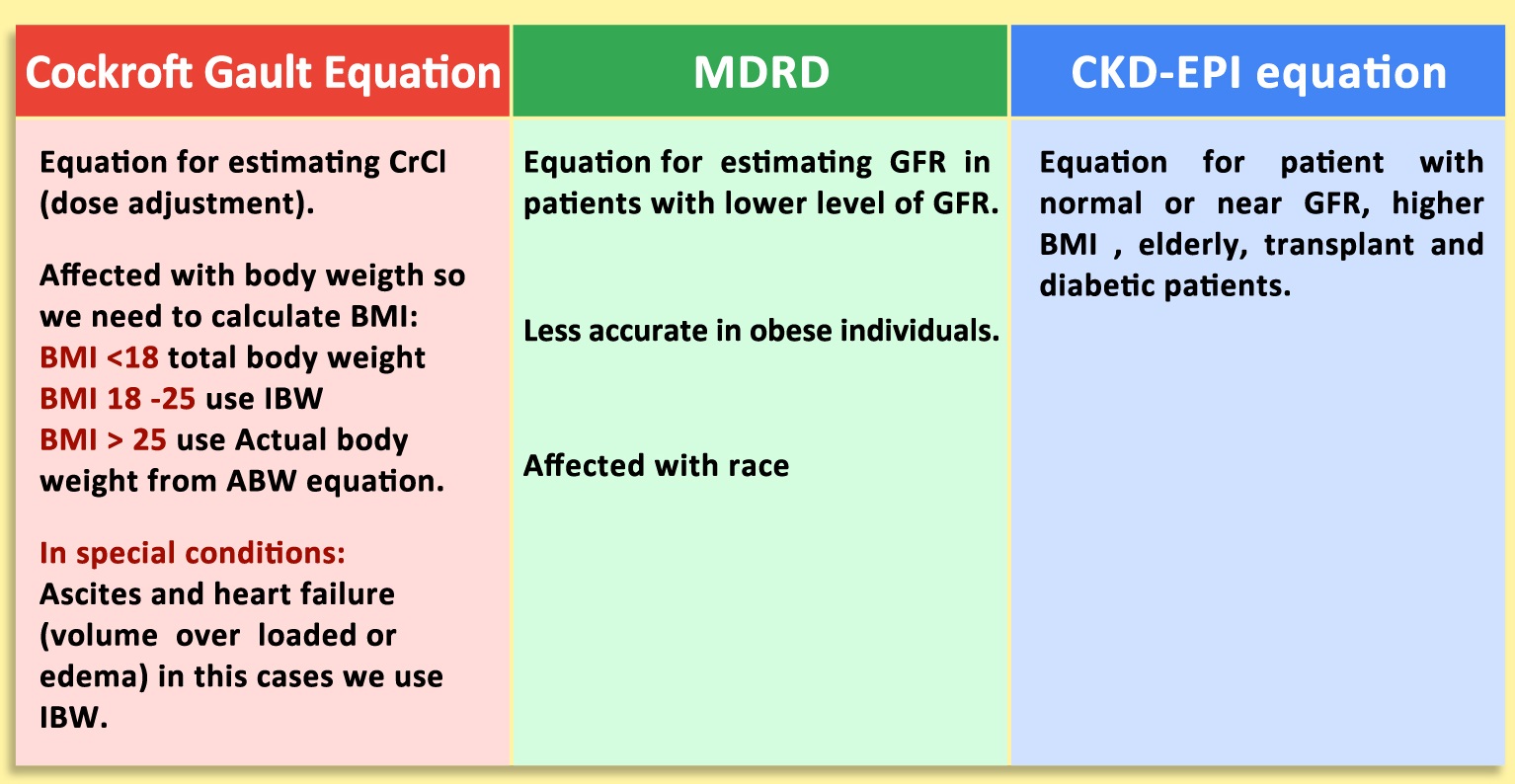
Compare Between MDRD, Cockroft-Gault Equation and CKD-EPI Equation
Creatinine clearance formula
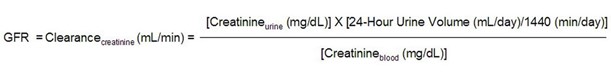
- Put differently, if the GFR decreases and production (and daily excretion) remains constant, the serum concentration has to increase.
- (The serum concentration increases until the concentration is such that, when multiplied by the GFR, the product matches the daily excretion.)
- Thus, if one loses half of one’s renal function, the creatinine doubles.
- As creatinine increase from 1.0 to 2.0 represents the same decrement in renal function as a creatinine increase from 2.0 to 4.0 or 4.0 to 8.0, as reflected in the graph below.
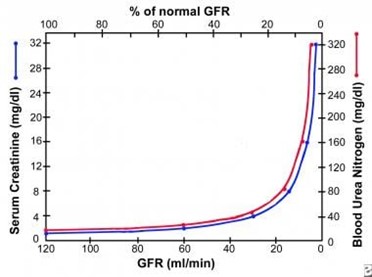
Indications/applications
- Because different individuals have different muscle masses, using a single reference interval can be misleading.
- A serum creatinine of 1.0 in a 50-year-old white woman represents a significantly reduced GFR; the same value in a 25-year-old black male represents a normal GFR.
- Determining GFR accurately requires onerous methods (intravenous infusions, esoteric laboratory measurements [inulin], and/or radioactive compounds.
- But a more practical way of determining the GFR has been the creatinine clearance.
- Furthermore, all this will require a 24-hour urine collection, urine and serum creatinine measurement, and the use of the formula above.
Considerations in Creatinine Clearance
- In 2006, a landmark study illustrated that one could calculate a reasonably good estimate of the GFR by using the serum creatinine, age, gender, and ethnicity of the patient.
- This equation named the MDRD equation because it was based on a study called the Modification of Diet in Renal Disease.
- A recommendation made and largely adopted that laboratories report an estimated GFR (eGFR) with every measured serum creatinine study.
- Although the eGFR has a few limitations, it works quite well, especially in detecting reduced GFR at early stages.
- Moreover, the eGFR promoted as a valuable tool in screening for CKD.
- One of its limitations is that the eGFR calculation is invalid in various situations.
- For example, (e.g., pediatric patients, very elderly patients, pregnant individuals, extremes of body habitus, malnutrition, paraplegia, patients with skeletal muscle disease, and those with rapidly changing kidney function).
CrCl Estimation Equations
Equations used in Creatinine Clearance
- Despite the MDRD equation used by most clinical laboratories for reporting eGFR in adults, several other equations for calculating GFR can be used, some of which should be mentioned here (e.g., CKD EPI, Bedside Schwartz, and Cockcroft-Gault).
- The MDRD equation was designed to detect reduced GFR, but it does not work well for values above 60 mL/min/1.73 m2, so labs typically report such values simply as “>60.”
- Using the same set of variables as the MDRD equation, the CKD EPI formula claims a higher level of accuracy with higher (normal) GFRs.
- Again, the NKDEP recommendation estimated GFRs reported automatically for adults.
- Moreover, when an estimation of GFR needed for pediatric patients, the MDRD equation not used.
- Instead, the Bedside Schwartz equation, based on the patient’s height and serum creatinine, is preferable.
- Last, although still in wide use, the Cockcroft-Gault equation superseded the newer equations already mentioned.
- Except for, notably, drug dosing. Because most drugs received FDA approval before the existence of the MDRD equation, their renal dosing guidelines typically based on the Cockcroft-Gault equation.
- Although controversial, the differences in GFR generated by the two equations are usually insignificant.
- When present, differences often ascribed to the fact that the assumptions underlying the Cockcroft-Gault equation not met, as follows:
- Current creatinine methods calibrated differently
Equations for GFR Calculation
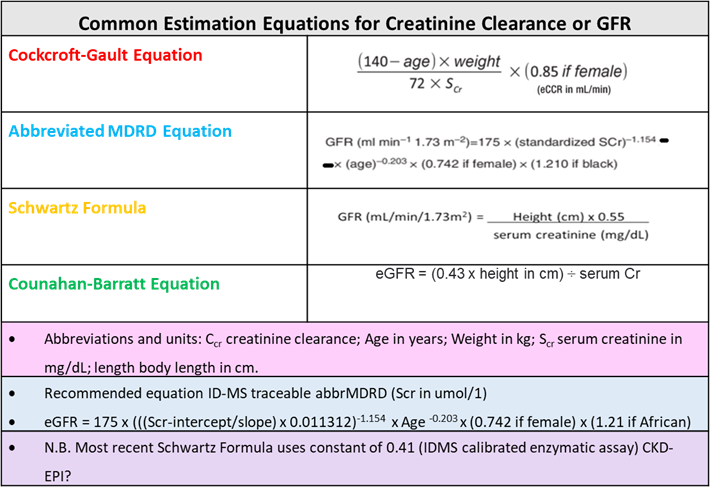
- Failure to use the patient’s “lean body mass.”
- Moreover, Creatinine is critically important in assessing renal function because it has several interesting properties.
- In addition, blood is a marker of glomerular filtration rate.
- In urine, it can remove the need for 24-hour collections for many analytes or used as a quality assurance tool to assess the accuracy of a 24-hour collection.
The reference ranges for serum creatinine are
Adult:
- Female: 0.5-1.1 mg/dL or 44-97 μmol/L (SI units)
- Male: 0.6-1.2 mg/dL or 53-106 μmol/L (SI units)
- Elderly: Reduced muscle mass may lead to lower values
- Adolescent: 0.5-1.0 mg/dL
- Child: 0.3-0.7 mg/dL
- Infant: 0.2-0.4 mg/dL
- Newborn: 0.3-1.2 mg/dL
- Possible critical values: >4 mg/dL (indicates seriously impaired renal function)
The reference interval varies with race, ethnicity, and gender.
As a result, one should look at the calculated eGFR (estimated glomerular filtration rate), as reported from the measured serum creatinine, to assess renal function.
The GFR can also be calculated from the creatinine clearance.
Interpretation of Creatinine Clearance.
- Low serum creatinine values are rare; they almost always reflect low muscle mass.
- Theoretically, low values may also reflect increased glomerular filtration rates (GFRs).
- Serum creatinine increases with decreases in GFR (acute kidney injury or chronic kidney disease).
- The increase in serum creatinine is relatively minor at the earliest stages of the disease due to mathematical reasons.
- For example (eg, a patient whose baseline creatinine is 0.6 would have to lose more than 50% of his GFR before the creatinine would increase to 1.3 and first be noted as “abnormal” by most reference intervals).
- Inter-individual differences in muscle mass related to gender, age, and ethnicity (among other things) are the cause.
- As a result, for adult patients (age 18 and over), along with every measured serum creatinine, most clinical labs now report an estimated GFR (eGFR).
- The eGFR accounts for some of these variables and can alert physicians to significant reductions in GFR even when the serum creatinine appears normal or only minimally elevated.
- However, note that the calculation is not valid in various situations (e.g., pediatric patients, very elderly patients, pregnant individuals, extremes of body habitus, malnutrition, paraplegia, patients with skeletal muscle disease, and those with rapidly changing kidney function).
- In some cases, more accurate information on the GFR obtained from a 24-hour creatinine clearance.
- Moreover, it requires an accurate 24-hour urine collection and urine serum creatinine measurement.
- All 24-hour urine collections should be assessed for accuracy/completeness by calculating the 24-hour total creatinine excretion.
Creatinine Clearance Calculator
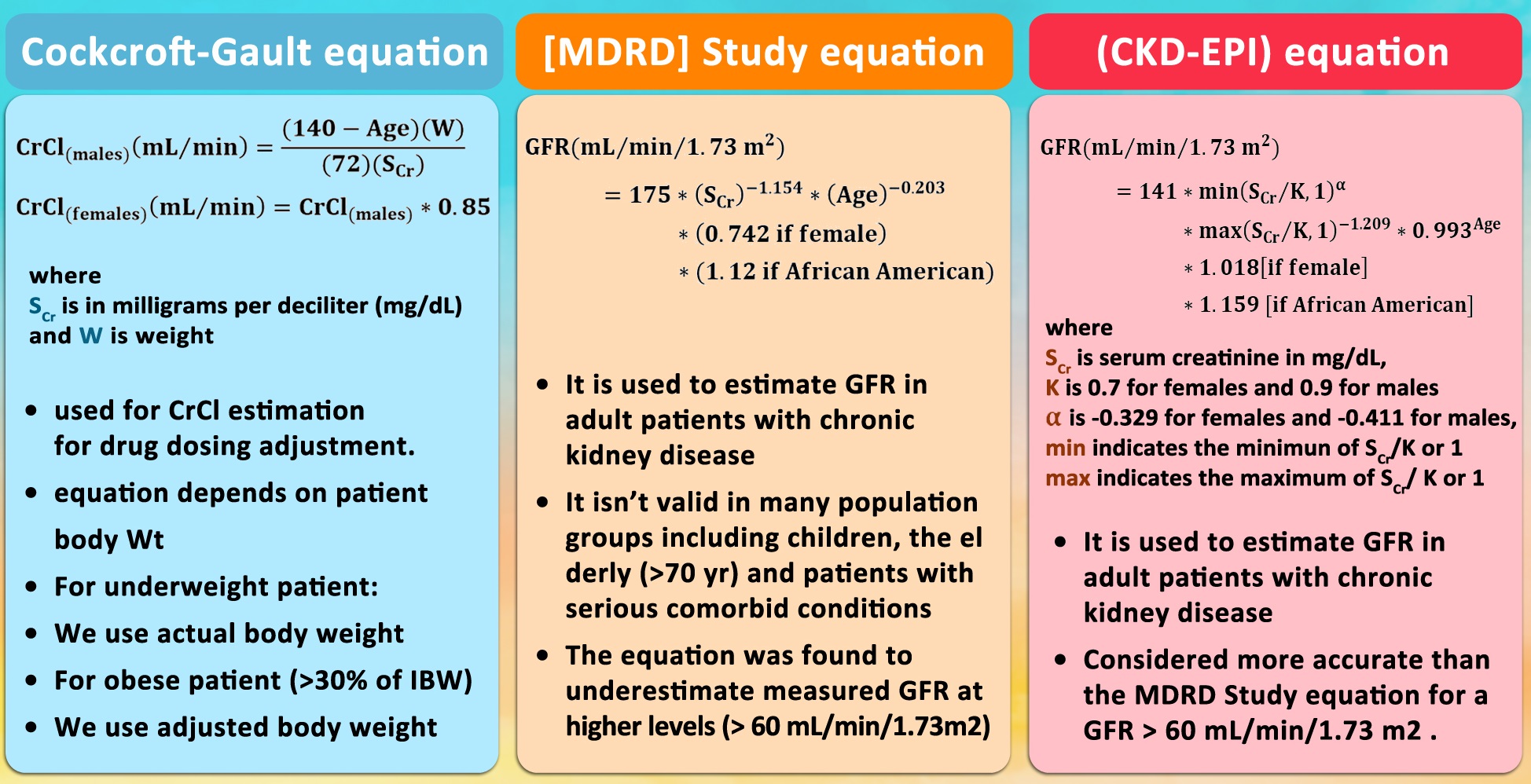
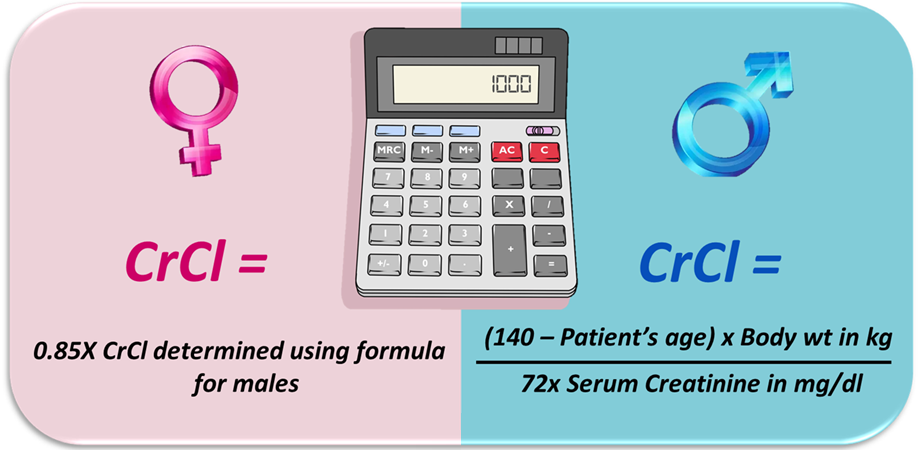
The Cockcroft-Gault formula:
Estimating creatinine clearance (CrCl) used routinely as a simple means to provide a reliable approximation of residual renal function in all patients with CKD.
The formulas are as follows:
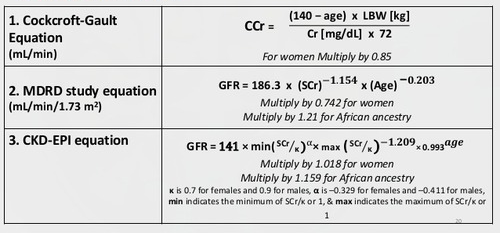
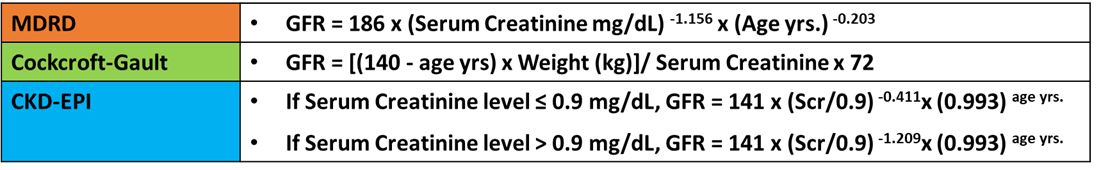
- CrCl (male) = ([140-age] × weight in kg)/(serum creatinine × 72)
- CrCl (female) = CrCl (male) × 0.85
Alternatively, the Modification of Diet in Renal Disease (MDRD) Study equation used to calculate the glomerular filtration rate (GFR).
Additionally, this equation does not require a patient’s weight.
Moreover, the MDRD underestimates the measured GFR at levels above 60 mL/min/1.73 m2.
However, a study al found that the CKD-EPI and MDRD equations underestimated GFR in patients with type 2 diabetes.
The measured GFR was 103 ± 23 mL/min/1.73 m², and the CKD-EPI GFR was 83 ± 15 mL/min/1.73 m².
Additionally, the MDRD GFR was 78 ± 17 mL/min/1.73 m². Accuracy was 67% for the CKD-EPI equation and 64% for the MDRD equation.
Cockcroft-Gault Equation
Renal function calculation in pediatric patients
- GFR in children is calculated using the Schwartz formula.
- Because this formula may currently overestimate GFR, likely due to a change in methods used to measure creatinine.
- Moreover, Schwartz et al. proposed an updated equation that includes cystatin C.
- However, the majority of dosing guidelines for medication adjustments due to reduced GFR use the original Schwartz equations.
Renal function calculation in elderly patients
- Age is an important consideration with respect to estimated GFR. In a 70-kg man aged 25 years, a serum creatinine value of 1.2 mg/dL represents an estimated GFR of 74 mL/min/1.73m2, but in a 70-kg man aged 80 years, that same value represents an estimated GFR of 58 mL/min/1.73m2.
- Thus, in a 70-kg, 80-year-old man, a serum creatinine of 2 mg/dL actually represents severe renal impairment, with an estimated GFR of 32 mL/min/1.73 m2 as measured by the MDRD equation.
- Therefore, in elderly patients an estimated GFR determined using a formula such as the MDRD equation, which includes age as a variable.
- This will allow appropriate adjustments of drug dosing and avoidance of nephrotoxins.
- In addition to, patients who have more extensive CKD suggested by the serum creatinine value alone.
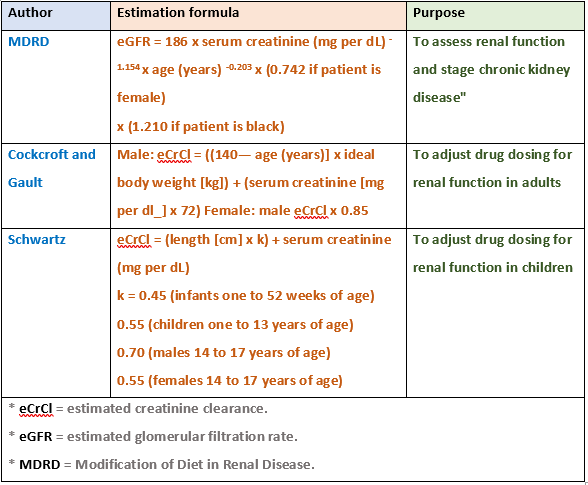
Drug Dosing According to Creatinine Clearance Calculation
- The MDRD Study equation and more recently the CKD-EPI equation have been shown to be more accurate than the Cockcroft and Gault equation
- The MDRD Study and CKD-EPI equations now more widely reported automatically by clinical laboratories every time a measured creatinine clearance.
- Creatinine assays standardised to reference methods.
- Both the MDRD Study and CKD-EPI expressed for these reference methods, but The Cockcroft and Gault has not.
- The assay used to develop the Cockcroft and Gault was likely 10-20% higher than current methods.
- Therefore, estimated creatinine clearance calculated using the Cockcroft and Gault will lead to higher drug dosing recommendations than intended in the original pharmacokinetic studies.
- Multiple studies compared the equations for their impact on drug dosages.
- In the few studies compared estimated GFR from the various equations to measured GFR.
- The studies have shown that the MDRD Study or CKD-EPI equation had greater concordance with measured GFR than the Cockcroft and Gault.
Selecting an Equation
- The Modification of Diet in Renal Disease (MDRD) Study equation and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation the most widely used IDMS traceable equations for estimating GFR in patients age 18 and over.
- In addition to, estimating GFR from serum creatinine in patients under age 18 (including infants, toddlers, children, and teens), the Bedside Schwartz equation used.
- Moreover, both the MDRD Study and CKD-EPI equations include variables for age, gender, and race, which may allow providers to observe that CKD is present despite a serum creatinine concentration that appears to fall within or just above the normal reference interval.
- Note that creatinine clearance considered for assessing kidney function when the patient’s basal creatinine production is very abnormal.
- Furthermore, this may be the case with patients of extreme body size or muscle mass (e.g., obese, severely malnourished, amputees, paraplegics, or other muscle-wasting diseases), or with unusual dietary intake (e.g., vegetarian, creatine supplements).
The Cockcroft-Gault formula
- The Cockcroft-Gault (CG) formula not expressed using standardised creatinine values. This means it will give inaccurate results. It is not recommended for clinical use.
- Moreover, Creatinine assays are standardised to reference methods.
- Both the MDRD Study and CKD-EPI expressed for these reference methods, but the Cockcroft-Gault formula has not.
- The assay used to develop the Cockcroft-Gault was likely 10-20% higher than current methods.
- Therefore use of estimated creatinine clearance calculated using the Cockcroft-Gault will lead to higher drug dosing recommendations than intended in the original pharmacokinetic studies.
- Multiple studies compared the equations for their impact on drug dosages.
- In the few studies that compared estimated GFR from the various equations to measured GFR.
- In addition, the studies have shown that the MDRD Study or CKD-EPI equation had greater concordance with measured GFR than the Cockcroft-Gault.
- Moreover, one study of inpatients receiving aminoglycoside or vancomycin compared the area under the curve for actual drug levels to the e-GFR and showed greater precision for the MDRD Study equation.
- Since the Cockcroft-Gault can overestimate kidney function, there is a risk of overdosing drugs with narrow therapeutic index that has occurred with chemotherapeutic agents.
The MDRD Equation
- The following is the IDMS-traceable MDRD Study equation (for creatinine methods calibrated to an IDMS reference method)
- GFR (mL/min/1.73 m2) = 175 × (Scr)-1.154 × (Age)-0.203 × (0.742 if female) × (1.212 if African American).
- Furthermore, the equation does not require weight or height variables because the results reported normalised to 1.73 m2 body surface area.
- Which is an accepted average adult surface area.
- Moreover, the equation validated extensively in Caucasian and African American populations between the ages of 18 and 70.
- Additionally, with impaired kidney function (eGFR < 60 mL/min/1.73 m2) and has shown good performance for patients with all common causes of kidney disease.
- Furthermore, the equation validated in patients older than 70, but an MDRD-derived eGFR may still be a useful tool for providers caring for patients older than 70.
The CKD-EPI Equation
- The CKD-EPI equation uses a 2-slope “spline” to model the relationship between GFR and serum creatinine, age, sex, and race.
- Moreover, this equation given in the following table for creatinine in mg/dL.
- Finally, the equation expressed in a single equation or as a series of equations for different race, sex, and creatinine conditions.
Estimation of GFR
Formulas used to estimate eGFR
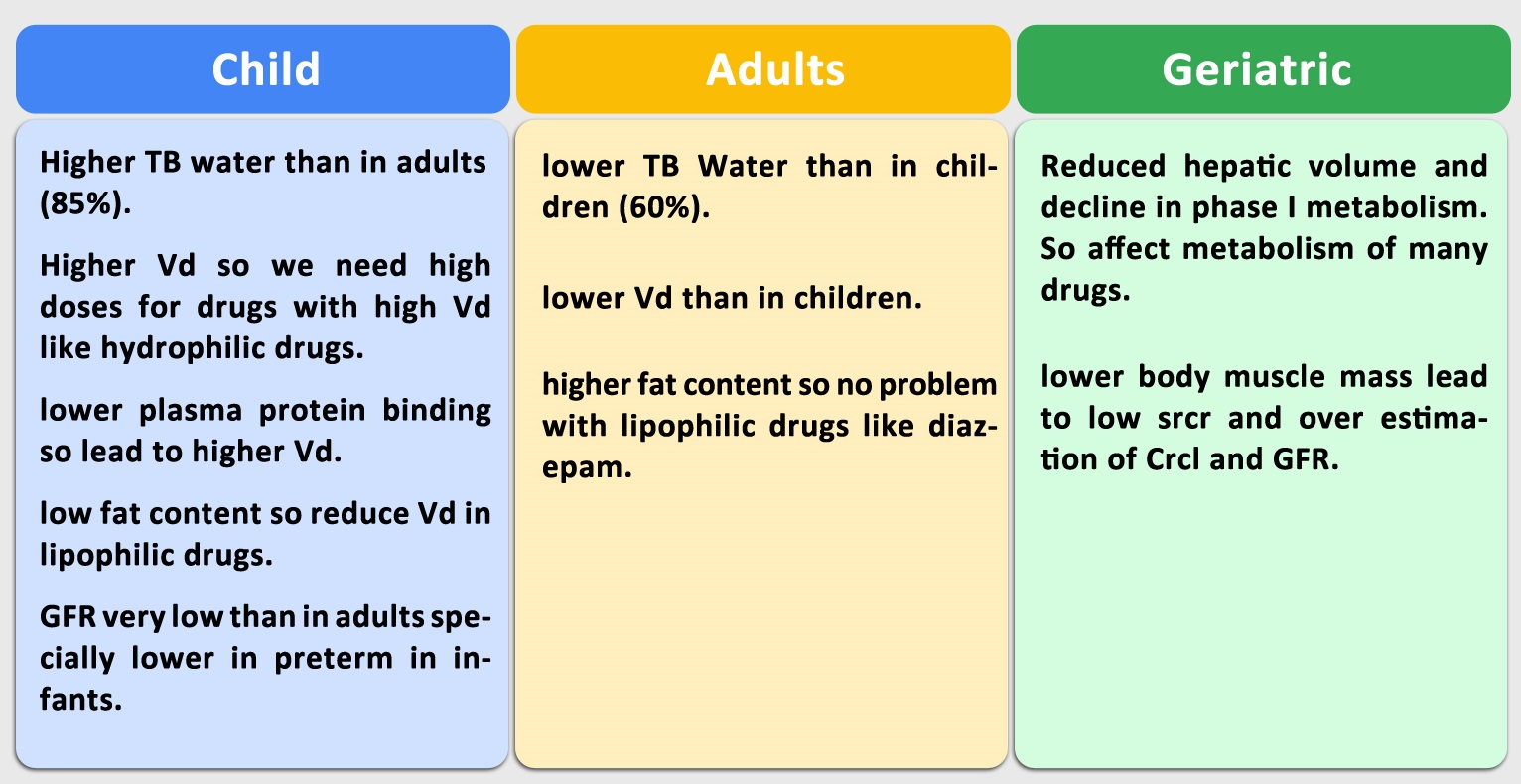
Difference Between Adults, Geriatrics and Child Pharmacokinetics
Read More:
- Antimicrobial Stewardship School
- Sepsis Training Program
- Download Pocket Guide for Antibiotic Pharmacotherapy Book
- Microbiology Course | ABC Bacteria
- Infectious Disease E-News | FREE Subscription
- ABC antimicrobials | Know all about the Antimicrobials
- Road Map to Antimicrobial Stewardship Training Program
- Register Now in FADIC Clinical Research School
- FADIC Drug Information Fellowship
- Buy FADIC Toolkit for Writing Research to Write a Great Research Paper
- Read 10 Skills You Must Learn to Make a Research via Google Scholar in Arabic
- The FADIC Online Continuous Medical Improvement Programs & Mini-Courses.
- Check Now FADIC Book store and Buy books in different specialties.
- Watch Now FADIC TV to Keep your self Updated.
- FADIC Podcast focusing on varieties of pharmacist perspectives in different specialties.
- Subscribe Now in FADIC 2020 Daily News (FNN) and Keep Updated.
- Check Now about Coronavirus Resource Information Center.

 Log in
Log in Sign up
Sign up