First Oral COVID-19 Antivirals
First Oral COVID-19 Antivirals
In the United States
Phase 2/3 results of PAXLOVID First Oral COVID-19 Antivirals
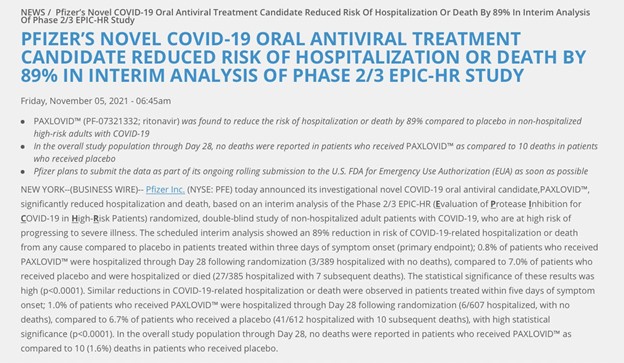
- Pfizer’s COVID-19 oral antiviral shows the risk of hospitalisation or death is REDUCED by NEARLY 90% in high-risk adults with COVID-19 when taken within three days of experiencing symptoms.
- This protease inhibitor was initially developed for SARS-COV in 2003 and has been repurposed!
- So first, the scheduled interim analysis showed an 89% reduction in risk of COVID-19-related hospitalisation or death from any cause compared to placebo in patients treated within three days of symptom onset (primary endpoint).
Pfizer says COVID-19 pill cut hospital, death risk by 90%
- While 0.8% of patients who received PAXLOVID™ hospitalised through Day 28 following randomisation (3/389 hospitalised with NO deaths).
- Compared to 7.0% of patients received placebo and hospitalised or died (27/385 hospitalised with seven subsequent deaths).
- In the overall study population through Day 28, NO deaths reported in patients who received PAXLOVID as compared to 10 deaths in patients who received placebo.
- PAXLOVID is an investigational SARS-CoV-2 protease inhibitor antiviral therapy.
- It specifically designed to be administered orally so that it can be prescribed at the first sign of infection or at first awareness of an exposure.
- Additionally, it is potentially helping patients avoid severe illness which can lead to hospitalisation and death.
How do First Oral COVID-19 Antivirals work exactly?
It is designed to block the activity of the SARS-CoV-2-3CL protease, an enzyme that the coronavirus needs to replicate.
The co-administration with a low dose of Ritonavir helps slow its metabolism of it to remain active in the body for more extended periods at higher concentrations to help combat the virus.
In addition, it inhibits viral replication at a stage known as proteolysis, which occurs before viral RNA replication.
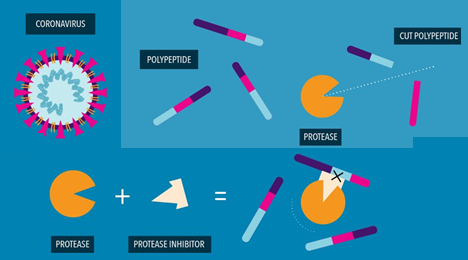
PROTEASE INHIBITORS TO FIGHT COVID-19:
STOPPING THE VIRUS’S LIFE CYCLE
- Researchers are studying protease inhibitors for their potential to stop the virus that causes COVID-19, from replicating. Here’s how these medicines work:
- The SARS.COV.2 virus, is a single-strand RNA wrapped in a protein envelope.
- The RNA Contains the genetic instructions the virus needs to make copies of itself
- When the Virus invades a human cell, its RNA translated into a polypeptide, a long protein chain that includes several enzymes necessary to continue replication.
- Before these enzymes can start working, they need to be separated from each other.
- The protease enzyme acts like a pair of scissors, cutting the polypeptide up into the different enzymes that become functional.
- Protease inhibitors can interfere with this critical cutting step.
- These drugs designed to tightly bind to the protease enzyme, blocking its ability to cut.
- The Virus then can’t make copies of itself and further infect its host.
First Oral COVID-19 Antivirals
- Pfizer’s pill, scientifically known as PF-07321332, is part of a class of medicines called protease inhibitors and works by inhibiting an enzyme the virus needs to replicate in human cells.
- Protease inhibitors used to treat other viral pathogens such as HIV and hepatitis C.
- The HIV drug helps slow the metabolism, or breakdown, of Pfizer’s pill to remain active in the body for more extended periods of time at higher concentrations.
- The company said its data on the drug is based on a mid-to-late stage study of 1,219 adults with at least one underlying medical condition and a laboratory-confirmed infection within five days.
- Participants also given a low dose of Ofritonavir, a medication commonly used in combination treatments for HIV.
- In preclinical studies, The PAXLOVID™ didn’t demonstrate any evidence of mutagenic DNA interactions.
- At the recommendation of an independent Data Monitoring Committee and in consultation with the U.S.
FDA and First Oral COVID-19 Antivirals Efficacy
- The Food and Drug Administration (FDA), Pfizer will cease further enrollment into the study due to the overwhelming efficacy demonstrated in these results.
- Additionally, the plans to submit the data as part of its ongoing rolling submission to the U.S. FDA for Emergency Use Authorisation (EUA) as soon as possible.
- If approved or authorised, PAXLOVID, which originated in Pfizer’s laboratories, would be the first oral antiviral of its kind, a specifically designed SARS-CoV-2-3CL protease inhibitor.
- Upon successful completion of the remainder of the EPIC clinical development program and subject to approval or authorisation.
- It prescribed more broadly as an at-home treatment to help reduce illness severity, hospitalisations, and deaths.
- As well as reduce the probability of infection following exposure, among adults.
- It demonstrated potent antiviral in vitro activity against circulating variants of concern.
- In addition to, the other known coronaviruses, suggesting its potential as a therapeutic for multiple types of coronavirus infections.
In the United Kingdom
First Oral COVID-19 Antivirals – Lagevrio (molnupiravir) – Approved by MHRA
- The antiviral Lagevrio (molnupiravir) is safe and effective at reducing the risk of hospitalisation and death in people with mild to moderate COVID-19 who are at increased risk of developing severe disease.
- The Medicines and Healthcare products Regulatory Agency (MHRA) announced this medication.
- This follows a rigorous review of its safety, quality and effectiveness by the UK regulator and the government’s independent expert scientific advisory body.
- The Commission on Human Medicines, making it the first oral antiviral for the treatment of COVID-19 to be approved.
First Oral COVID-19 Antivirals in the UK
- Developed by Ridgeback Biotherapeutics and Merck Sharp & Dohme (MSD).
- The Lagevrio works by interfering with the virus’ replication.
- This prevents it from multiplying, keeping virus levels low in the body and reducing the disease’s severity.
- Based on the clinical trial data, Lagevrio is most effective when taken during the early stages of infection.
- Additionally, the MHRA recommends its use as soon as possible following a positive COVID-19 test and within five days of symptoms onset
- Molnupiravir authorised for use in people who have mild to moderate COVID-19 and at least one risk factor for developing severe illness.
- Such risk factors include obesity, older age (>60 years), diabetes mellitus, or heart disease.
- It’s now the second antiviral pill behind Merck’s to demonstrate strong effectiveness for treating Covid at the first sign of illness which the MHRA just approved
“This antiviral will be an excellent addition to our armoury against COVID-19, and it remains vital everyone comes forward for their life-saving COVID-19 vaccine – particularly those eligible for a booster – to ensure as many people as possible are protected over the coming months.”
The COVID-19 Vaccination Still Very Important Preventative
- Importantly, these PAXLOVID™ or LAGEVRIO™ not meant to replace the COVID-19 vaccines.
- The Vaccination is still our best tool to reduce the risk of infection, hospitalisation, and death due to COVID-19.
- If infected patient, whether due to being unvaccinated OR a “breakthrough” infection.
- Additionally, when you are experiencing symptoms.
- These antivirals can help immediately reduce hospitalisation risk even further.
Read More:
- Antimicrobial Stewardship School
- Sepsis Training Program
- Download Pocket Guide for Antibiotic Pharmacotherapy Book
- Microbiology Course | ABC Bacteria
- Infectious Disease E-News | FREE Subscription
- ABC antimicrobials | Know all about the Antimicrobials
- Road Map to Antimicrobial Stewardship Training Program
- Register Now in FADIC Clinical Research School
- FADIC Drug Information Fellowship
- Buy FADIC Toolkit for Writing Research to Write a Great Research Paper
- Read 10 Skills You Must Learn to Make a Research via Google Scholar in Arabic
- The FADIC Online Continuous Medical Improvement Programs & Mini-Courses.
- Check Now FADIC Book store and Buy books in different specialities.
- Watch Now FADIC TV to Keep Yourself Updated.
- FADIC Podcast focusing on varieties of pharmacist perspectives in different specialities.
- Subscribe Now to FADIC 2020 Daily News (FNN) and Keep Updated.
- Check Now about Coronavirus Resource Information Center.

 Log in
Log in Sign up
Sign up