Sofosbuvir Between the Hope and the Fact
Sofosbuvir Between the Hope and the Fact
Hepatitis C virus (HCV)
- Hepatitis C is a disease with a significant global impact. Moreover, given many names, ‘the silent epidemic’, ‘the silent dragon’ and ‘the disease of the new millennium (1).
- In addition to, the Hepatitis C virus (HCV) is one of the most important Flaviviridae infections in humans the second most common cause of viral hepatitis.
- Finally, the Most patients fail to clear the virus and develop persistent infection that frequently leads to cirrhosis and hepato-cellular carcinoma (HCC) (2).
Properties of HCV
- HCV is a positive-stranded RNA virus, classified as family Flaviviridae, genus Hepacivirus.
- Additionally, the HCV can be differentiated by RNA sequence analysis into at least six major genotypes (clades) and more than 70 subtypes (3)
- In addition to, the HCV infects only humans and chimpanzees, primarily targeting hepatocytes. Apart from hepatocytes.
- Moreover, there is strong evidence that HCV can also replicate in peripheral blood mononuclear cells (PBMCs) both in vivo and ex vivo or in experimentally infected B- and T-cell lines.
- Viral replication is extremely robust, and estimated that more than 10 trillion virion particles produced per day, even in the chronic phase of infection(4).
Structure of HCV Virions
- The hepatitis C virus particle consists of a core of genetic material RNA surrounded by an icosahedral protective shell of protein, and further encased in a lipid (fatty) envelope of cellular origin. Two viral envelope glycoproteins, E1 and E2, are embedded in the lipid envelope(5).
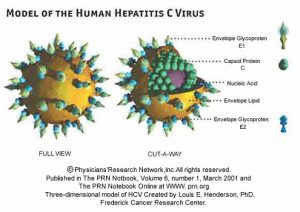
Three –dimension model of HCV
Viral genome
- The genome of HCV is approximately 9600 nucleotides long . It contains two highly conserved untranslated regions (UTR) 5’-UTR and 3’-UTR that are flanking a single open reading frame (ORF). Dependent on the genotype, the ORF might contain from 9030 to 9099 nucleotides and it is coding for a single polyprotein
- precursor of 3010 to 3033 amino acids (aa), respectively. Translation occurs in the endoplasmic reticulum and it is initiated by IRES at the 5’ UTR.(6)
Viral proteins
- The translation occurs in the endoplasmic reticulum and it is initiated by IRES at the 5’UTR. A single polyprotein precursor is processed by cellular and viral proteases into ten proteins . Three structural proteins (core, E1, E2) are located at the amino-terminal part of the polyprotein and are essential components of the virions.
- Seven nonstructural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A, NS5B) are located in the remaining part of the polyprotein and these proteins are involved in particle morphogenesis, RNA replication and in regulation of cell functions.It is worth mentioning, that the structural and non-structural proteins of HCV are multifunctional. (6)
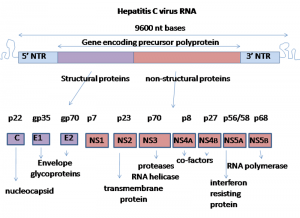
(Graham Colm, 2007).
| FUNCTIONS | DEFINITION | REGION |
| The 5′ UTR has a ribosome binding site (IRES – Internal ribosome entry site) that starts the translation | it is 5 end of the RNA of HCV | 5′ UTR |
– forms the viral nucleocapsid-interact with numerous cellular proteins and affect host cell functions |
It is a highly basic, RNA-binding protein | Core |
| E1 and E2 form non-covalent heterodimers which are believed to represent the building blocks for the viral envelope. | The envelope glycoproteins E1 and E2 are type I transmembrane proteins with C-terminal hydrophobic anchors. | E1 |
| E2 | ||
| The ARFP/F protein is dispensable for HCV RNA replication. Whether it is expressed during natural HCV infection has still to be clarified. | It is a protein encoded by an alternative reading frame within the core region and comprises up to 160 amino acids. | ARFP/F protein
ARFP (alternative reading frame protein) F (frameshift) protein |
| It is believed that p7 could be important
for viral assembly |
p7 is a 63-amino acid polypeptide located at the
junction between the structural and nonstructural region. |
P7 |
| It is unclear whether NS2 fulfills any further functions after separation from NS3 | It is a non-structural regulatory protein which have NS2/3 junction cleaved by a remarkable autoprotease consisting of NS2 and the N-terminal third of NS3. | NS2 |
| NS3 is a multifunctional protein because it harbors a 1-serine protease located in the N-terminal one-third that is responsible for the downstream cleavage in the nonstructural region and the NS3 serine protease recently turned out to influence the innate cellular host defense by inhibition of RIG-I and TLR3 signalling 2-NTPase/RNA helicase domain
in the C-terminal two-thirds. |
It is a non-structural regulatory protein | NS3 |
It targets NS3 to intracellular membranes andis required as a cofactor for the NS3 serine protease |
It is a non-structural regulatory protein consisting of 54-amino acid polypeptide, | NS4A |
| The expression of NS4B induces a specific membrane alteration, designated as membranous web, that serves as a scaffold for the formation of the viral replication complex | It is very hydrophobic integral membrane protein that localizes to an ER derived membranous compartment |
NS4B |
| NS5A phosphorylation has an impact on replication efficiency, these observations support the concept that NS5A plays an important role in the
regulation of viral replication and in modulating the IFN response |
NS5A is a phosphorylated zinc metalloprotein | NS5A |
The key enzyme of the replicase that promotessynthesis of new RNA genomes is the NS5B RNAdependent RNA polymerase (RdRp). |
NS5B is a tailanchored protein, characterized by a transmembrane domain at the C-terminus of the protein responsible for posttranslational membrane targeting | NS5B |
| critical regulation in virus replication | it is 3 end of the RNA of HCV | 3-UTR |
The HCV life Cycle:
- The investigation of the HCV life cycle and pathogenesis has been complicated by the lack of efficient cell culture systems and small animal models (7).
- The Key steps in the life cycle of HCV include entry into the host cell, uncoating of the viral genome, translation of viral proteins, viral genome replication, and the assembly and release of virions. all these events occur outside the nucleus of the host cell (8)
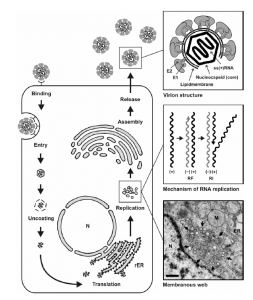
Hypothetical HCV replication cycle
HCV Bind to Host Cells
- HCV particles bind to the host cells via interaction between the HCV envelope glycoproteins and a cellular receptor.
- Bound particles are probably internalized by receptor-mediated endocytosis.
- After the viral genome is liberated from the nucleocapsid (uncoating).
- Then, translated at the rough ER, NS4B induces the formation of membranous vesicles.
- These membranes are supposed to serve as scaffolds for the viral replication complex.
- After genome implication and HCV protein expression, progeny virions are assembled.
- Newly produced virus particles may leave the host cell by the constitutive secretory pathway.
N: nucleus; ER: endoplasmic reticulum; M: mitochondria.
Route of Transmission
- blood transfusions or other blood products,
- medical procedures : the reuse of medical instruments used in invasive settings (e.g., needles, infusion sets, syringes, catheters )
- injection drug use.
- organ transplantation,
- hemodialysis, endoscopy
- Sexual or Household Contact: Usual household contacts do not pose a risk of HCV transmission.
The HCV Transmission
- The efficiency of HCV transmission by sexual contact is very low (0.4 to 3%).
- However, there is no doubt that sexual transmission of hepatitis C is possible.
- Nevertheless, the risk may increase when favoring conditions such as sexually transmitted infections
- The rate of HCV mother-to-child transmission is about4.3%, but it is much higher, 22.1% among the human immunodeficiency virus (HIV) co-infected mothers.
- Cosmetic procedures and/or acupuncture, piercings, tattooing as well as circumcision are extensively associated with HCV transmission, but the risk seems to be small (6,9)
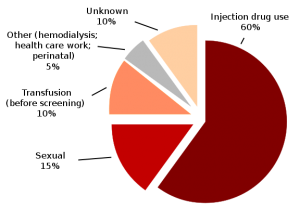
Exploded pie chart of Hepatitis C infection by source based on CDC data
(CDC figures, 2009).
Clinical manifestations and natural history of HCV infection
- The spectrum of clinical manifestations of HCV infection varies in acute versus chronic disease.
- Acute infection with HCV is most often asymptomatic. It leads to chronic infection in about 80% of cases.
- The manifestations of chronic HCV range from an asymptomatic state to cirrhosis, and hepato-cellular carcinoma. HCV infection usually is slowly progressive. (10)
- There is evidence that a subgroup of patients with chronic HCV infection may develop systemic and extra-hepatic manifestations which include chronic fatigue.
- In addition to reduced quality of life scores, cryoglobulinemia, vasculitis, low grade B cell lymphoma and a number of other, less well characterised clinical conditions(11)
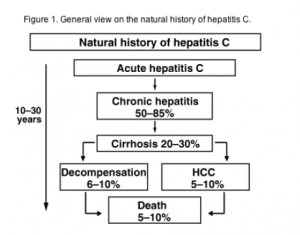
General view on the natural history of hepatitis C
(Alberti et al., 2005)
The Treatment for Chronic Hepatitis C
- The primary goal of treatment for chronic hepatitis C is a sustained virological response (SVR) which means the eradication of HCV infection.
- SVR 12 is defined as the absence of detectable HCV RNA in the blood at least 12 weeks after treatment completion.
- Achieving the endpoint of SVR associated with persistent regression of hepatic fibrosis, reduced incidence of cirrhosis, HCC and liver-related mortality (12).
- In the last two decades, treatment of the HCV infection significantly improved.
- For nearly 15 years, the combination of pegylated interferon alfa and ribavirin allowed a moderate sustain virologic response (SVR).
- Cure of the infection achieved in 45% of genotype 1 and 65% of genotype 3 and around 85% of genotype 2 infected patients (13).
Development of Sofosbuvir
- Development of the direct-acting antiviral drugs (DAAs) targeting viral proteins (NS3/4A protease, NS5B polymerase with nucleotide and non-nucleotide inhibitors, NS5A viral replication complex) was achieved due to the better understanding of the HCV life cycle.
- It revolutionised the treatment of chronic hepatitis C (14).
- From 2015 the standard of care is a combination of DAAs. Sofosbuvir, daclatasvir and the sofosbuvir/ledipasvir combination are part of the preferred regimens in the WHO guidelines, and can achieve cure rates above 95% (15)
- In 2018 and onwards, because of their virological efficacy, ease of use, safety and tolerability.
- DAA-based regimens are the best options in HCV-infected patients. Patients without cirrhosis and with compensated and decompensated cirrhosis can be treated with DAA including ‘‘treatment-naïve” patients (patients who have never been treated for their HCV infection) and ‘‘treatment-experienced ‘‘patients (patients who were previously treated with elder regimes). (16)
Sofosbuvir: The New Era
What is Sofosbuvir?
- Sofosbuvir is FDA approved nucleotide analog. Its chemical name is L-Alanine, N-[[P(S),2′R]-2′-deoxy-2′-fluoro-2′-methyl-P-phenyl-5′-uridylyl]-, 1-methylethyl ester and a molecular formula of C22H29FN3O9P.
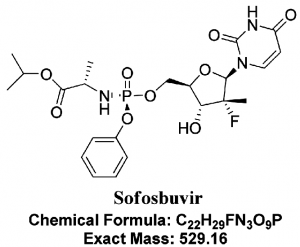
- It is a highly potent inhibitor of the NS5B polymerase in the Hepatitis C virus and has shown high efficacy in combination with several other drugs, with and without PEG-INF, against HCV.
- It offers many advantages due to its high potency, low side effects, oral administration, and high barrier to resistance. (17)
Mechanism of Action of Sofosbuvir
- NS5B is one of the non-structural proteins essential for viral RNA replication and has been found to be a valuable target for directly acting antiviral agents (DAAs).
- The uridine nucleotide analog sofosbuvir is a phosphoramidate prodrug that has to be triphosphorylated within the cells to produce its action.
- The required enzymes for its activation are present in the human hepatic cells.
- Therefore, it is converted to its active metabolite during the first-pass metabolism, directly at the desired site of action.
- This analog then mimics the physiological nucleotide and competitively blocks the NS5B polymerase, thus inhibiting the HCV-RNA synthesis by RNA chain termination.
- The catalytic site of the enzyme is also highly conserved across all the HCV genotypes, accounting for pan-genotypic efficacy of sofosbuvir.(17)
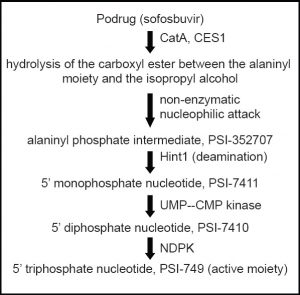
Activation of sofosbuvir in liver
- CatA -human cathepsin A; CES1- carboxylesterase 1; Hint1 – histidine triad nucleotide-binding protein 1; NDPK – nucleoside diphosphate kinase; UMP–CMP kinase-uridine monophosphate–cytidine monophosphate kinas
Pharmacokinetics of Sofosbuvir
- Sofosbuvir should be administered at the dose of 400 mg (one tablet) once per day, with or without food. Approximately
- 80% of sofosbuvir is renally excreted, whereas 15% is excreted in faeces.
- The majority of the sofosbuvir dose recovered in urine is the dephosphorylation-derived nucleoside metabolite GS-331007 (78%), while 3.5% is recovered as sofosbuvir.
- Renal clearance is the major elimination pathway for GS-331007, with a large part actively secreted.
- Thus, currently, no sofosbuvir dose recommendation can be given for patients with severe renal impairment.
- Sofosbuvir exposure is not significantly changed in patients with mild liver impairment, but it is increased 2.3-fold in those with moderate liver impairment (16)
- Sofosbuvir can be taken alone (Sovaldi®) or present in combinations with other DAA s as
Drug Interactions of Sofosbuvir
- Sofosbuvir not metabolized by cytochrome P450 but is transported by P-gp.
- In addition to, the Drugs that are potent P-gp inducers significantly decrease sofosbuvir plasma concentrations and may lead to a reduced therapeutic effect.
- Thus, sofosbuvir not be administered with known inducers of P-gp, such as:
- rifampicin,
- carbamazepine,
- phenytoin or St.
- John’s wort.
-
Other potential interactions may occur with
- rifabutin,
- rifapentine
- No significant drug-drug interactions reported in studies with the antiretroviral agents emtricitabine, tenofovir, rilpivirine, efavirenz, darunavir/ritonavir and raltegravir.
- In addition to, there are no potential drug-drug interactions with other antiretrovirals. (13)
Contraindication
- Sofosbuvir-based regimens contraindicated in patients
- who are being treated with the anti-arrhythmic amiodaron because of the risk of life-threatening arrhythmias.
- Indeed, bradycardia has been observed within hours to days of starting the DAA, but cases have been observed up to 2 weeks after initiating HCV treatment.
- Additionally, the mechanism of interaction and the role of other co-medications (e.g. b-blockers) is still unclear.
- If the patient has no cardiac pacemaker in situ, waiting 3 months after discontinuing amiodarone before starting a sofosbuvir-based regimen is recommended. (13)
Side effect
- Sofosbuvir well tolerated over 12 to 24 weeks of administration. The most common adverse events (≥20%) observed in combination with ribavirin were fatigue and headache.
- Slight elevations of creatine kinase, amylase and lipase without clinical impact were also observed. (13)
HCV & HCC: deep discussion
- The HCV is a leading cause of HCC worldwide and the morbidity and mortality from HCV-associated HCC is increasing, especially in high-income areas.
- Moreover, the HCC occurs mainly at patients with cirrhosis, but there is considerable heterogeneity in risk.
- The risk related to the severity of fibrosis, gender, age, diabetes and alfa-fetoprotein level at treatment among other factors.
- Several large cohort studies and meta-analyses examined the relationship between SVR and reduction in the risk of HCC.
- They show that SVR is associated with a substantial reduction in the incidence of HCC in the mid- to long-term. (13).
What studies Said?
- In a retrospective cohort study, done by researchers in Massachusetts General Hospital, Boston and Hamad Medical Corporation, Doha examined the risk and determinants of HCC patients cured with DAA based treatments.
- The outcome examined in 17,836 HCV-positive patient. The SVR achieved by 66.6% in patients treated with interferon (IFN)-based therapies, and by 96.2% in patients treated with DAA-based therapies.
- In addition to the data showed that IFN improved the outcomes following ablation or resection of HCC.
- They also reported that DAA treatment not associated with a higher risk of HCC in persons with cirrhosis with chronic HCV infection in the short term. (18)
- However, unexpectedly frequent early HCC recurrence with a more aggressive course reported in two retrospective studies in patients with HCV-related HCC.
- These studies who underwent curative procedures and subsequently treated with IFNfree regimens and cured from HCV in most cases. (19,20)
The Statistical Analysis
- The statistical analysis examined and the data criticised.
- Definite estimate of the HCC recurrence is difficult due to the high clinical biological and epidemiological heterogeneity of HCC.
- Also, the small number of patients and the lack of control arms are two of the most criticises.
- Moreover, the authors themselves concluded that their observation should be taken as a note of caution and should prime a larger scale assessment.13)
- Others criticised the studies and explain the reported higher rates of HCC associated with DAA treatment by both the presence of relatively fewer baseline HCC risk factors in persons treated with IFN.
- Furthermore, the selection bias, given that DAA regimens used to treat persons at higher risk for developing HCC.
DAA and HCC: scientific approach
- As we discussed in the previous section the confused relation between using DAA in treatment of chronic HCV and recurrence or incidence of HCC.
- Several studies suggest an increase in HCC recurrence or de novo incidence after DAA-induced SVR, whereas others do not report any change.
- In that section we will show different studies which support or against that thoughts.
- The first study was done by Conti F et al ,2016.
- The aim of this study was to evaluate the early occurrence and recurrence of HCC in cirrhotic patients treated with DAA.
- In patients with HCV-related cirrhosis
- DAA-induced resolution of HCV infection not seem to reduce occurrence of HCC.
- Additionally, patients previously treated for HCC have still a high risk of tumor recurrence, in the short term. (19)
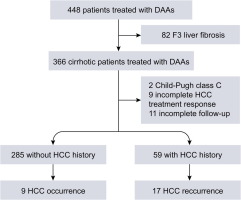
The Second Study
The second study we will discuss done by Reig M et al, 2016.
The study aimed to identify the effect of hepatitis C virus (HCV) eradication by using DAA- treatments in patients who have already developed hepatocellular carcinoma.
- Patients who receiving interferon as part of the antiviral regimen excluded.
- The data showed an unexpected high rate and pattern of tumor recurrence coinciding with HCV clearance.
- The authors themselves reported the fact that the data obtained based in a very small cohort of patients.
- In addition to the recommended to take that findings as a note of caution and prime a large-scale assessment that exceeds the individual investigators capacity.
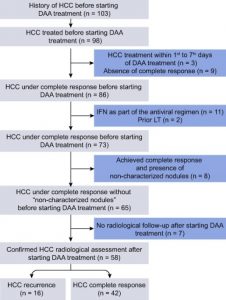
The Third Study
- The third study done by Ravi S et al,2016.
- Moreover, the study performed to examine the occurrence of de novo HCC among HCV-infected patients with cirrhosis during or after treatment with DAA.
- For final analysis the researchers used the data obtained only from 57 patients.
Conclusion from the study
- The study concluded that DAA therapy seems to increase the de novo occurrence of HCC among patients with HCV-related cirrhosis.
- On the other hand, they could not give an explanation for this.
- However, they recommended for Patients with HCV cirrhosis receiving DAA therapy, more rigorous and closer follow-up for surveillance of HCC.
- The authors mention some limitations for their work because of its retrospective design and selection bias given that 57 of the initial 123 patients excluded from the analysis. (21)
- The fourth study done by Kozbial K et al,2016.
Other Investigator Said
- Like the other investigators, they investigated the occurrence of hepatocellular carcinoma (HCC) in patients with advanced liver disease shortly after DAA-combination therapy in 16 without and in 3 with previous HCC.
- They also observed an increase in occurrence of HCC and also could not give a clear explanation for these observations.
- In addition to, they could not decide if this high number of HCC cases was due to a possible relation of HCC with IFN-free DAA therapy.
- Moreover, it occurred just by chance in temporal relation to antiviral therapy as a suspected end for HCV infection (22)
- All the previous studies mentioned in the last ESAL recommendation for 2018.
- In addition to, they make a lot of confusion among physicians, researchers and even patients.
Another study said
- Another important study done by Saraiya N et al, 2018.
- The study aimed to Characterize HCC recurrence patterns after DAA therapy by analyzing the results of 24 different studies (n = 1820 patients).
- In addition to the studies evaluating HCC recurrence patterns following DAA therapy published in MEDLINE and SCOPUS from January 2015 to December 2017.
- A pooled estimate calculated using the DerSimonian and Laird method for a random effects model.
- The study conducted in accordance with PRISMA guidelines. Even the data characterizing HCC recurrence after DAA therapy of limited quality.
- Furthermore, highlighting the need for high quality prospective studies.
- For the current data suggest acceptable HCC recurrence rates after DAA therapy, particularly if DAA therapy delayed at least 6 months after HCC complete response. (23)
- On the other hand, a lot of other study showed other opinions about the relation between HCC and DAA therapies.
- That studies also mentioned in ESAL recommendation for 2018.
- The first study performed by ANRS (French Agency for Research on AIDS and Viral Hepatitis.
Result Analysis
- In this study, they analyzed the data individually from three French prospective multicentre ANRS cohorts including more than 6000 patients treated with DAA.
- In addition to the focused on HCC patients who underwent curative procedures before DAA treatment.
- The aim was to assess the rates of HCC recurrence in these patients according to antiviral treatment regimen.
- They did not observe an increased risk of HCC recurrence after DAA treatment, notably in patients who underwent curative HCC treatment including liver transplantation (24)
- The second study done by Mettke F et al ,2018 and funded by German Center for Infection Research.
- The study aimed to investigate the HCC incidence in cirrhotic HCV patients who cleared HCV with direct-acting antivirals vs untreated controls by monitoring 373 patients.
- They concluded that DAA therapy of chronic hepatitis C does not alter the short-term risk for HCC in patients with liver cirrhosis.
- A reduced HCC incidence may become evident after more than 1.5 years of follow-up. (25)
- The third study performed by Virlogeux V et al, 2017.
- The study investigated the impact of DAA exposure on hepatocellular carcinoma recurrence after a first remission in patients with advanced fibrosis.
- They concluded that the HCC recurrence rate significantly lower among patients treated with DAA compared with untreated patients.
- In addition to, recommended large-scale prospective cohort studies to confirm these results.(26)
The Last Conclusion
- we can conclude From the different data mentioned before almost all studies suggested a decreased in incident hepatocellular carcinoma (HCC) after direct-acting antivirals (DAA).
- However, the data are conflicting regarding HCC recurrence and aggressiveness in patients who have a history of HCC with complete response.
- So that more attention and studies performed to find a clear recommendation about using DAA in treatment of HCV.
References
- World Health Organization. Hepatitis C. Fact sheet Number 164, revised October 2000. Available from: http://www.who.int/mediacentre/factsheets/fs164/en
- Yasmeen A. Hepatitis C Virus Persistence:Virus and Host Related Factors .2004 ;26-30
- Brooks GF, Butel JS , Morse SA . World Health Organization. Hepatitis C. Fact sheet. Medical Microbiology .2004;466:469.
- Bartenschlager R and Lohmann V. Replication of hepatitis C virus. J Gen Viro 2000; 81:1631-1648.
- Op De Beeck A, Dubuisson J. Topology of hepatitis C virus envelope glycoproteins. Rev. Med. Virol 2003 ; 13 (4): 233–4.
- Morozov VA et al . HCV: Morphogenesis, infection and therapyWorld J Hepatol 2018; 10(2): 186-212
- Brass V, Moradpour D and Blum E. Molecular Virology of Hepatitis C Virus (HCV): 2006 Update. International Journal of Medical Sciences 2006; 3(2):29-34.
Recommended Readings:
- Wasmuth JC.hepatology clinical textbook , Hepatitis C – Epidemiology, transmission and natural history 2010;19:30
- Abonyi M .diagnosis and treatment of chronic hepatitis c and its potential role in the pathogenesis of hepatocellular carcinoma.2001;1:7.
- Maylin S, Martinot-Peignoux M, Moucari R et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology 2008; 135: 821–9.
- EASL Clinical Practice Guidelines: management of hepatitis C virus infection.European Association for the Study of the Liver. J Hepatol. 2011 Aug; 55(2):245-64.
- Feeney ER, Chung RT, Antiviral treatment of hepatitis C. BMJ. 2014 Jul 7; 348():g3308
- Mauss S, Berg T, Rockstroh J, Sarrazin C, Wedemeyer H.Hepatology – Clinical textbook. 8th Edition. Hamburg: Medizin Fokus Verlag; 2017
- EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol (2018),
- ***Bhatia HK, Singh H, Grewal N, Natt NK. Sofosbuvir: A novel treatment option for chronic hepatitis C infection. Journal of Pharmacology & Pharmacotherapeutics. 2014;5(4):278-284. doi:10.4103/0976-500X.142464.
More To Read
- Kozbial K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon- free direct-acting antiviral treatment. J Hepatol 2016;65:856–858.
- Saraiya N1, Yopp AC2, Rich NE1, Odewole M1, Parikh ND3, Singal AG Systematic review with meta-analysis: recurrence of hepatocellular carcinoma following direct-acting antiviral therapy Aliment Pharmacol Ther.2018 May 30. doi: 10.1111/apt.14823.
- ANRS Collaborative Study Group on Hepatocellular Carcinoma. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol2016;65:734–740
- Mettke F, Schlevogt B, Deterding K, Wranke A, Smith A, Port K, et al. Interferon-free therapy of chronic hepatitis C with direct-acting antivirals does not change the short-term risk for de novo hepatocellular carcinoma in patients with liver cirrhosis. Aliment Pharmacol Ther 2018;47:516–525.
- Virlogeux V, Pradat P, Hartig-Lavie K, Bailly F, Maynard M, Ouziel G, et al. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int 2017;37:1122–1127.
Read More:
- Antimicrobial Stewardship School
- Sepsis Training Program
- Download Pocket Guide for Antibiotic Pharmacotherapy Book
- Microbiology Course | ABC Bacteria
- Infectious Disease E-News | FREE Subscription
- ABC antimicrobials | Know all about the Antimicrobials
- Road Map to Antimicrobial Stewardship Training Program
- Register Now in FADIC Clinical Research School
- FADIC Drug Information Fellowship
- Buy FADIC Toolkit for Writing Research to Write a Great Research Paper
- Read 10 Skills You Must Learn to Make a Research via Google Scholar in Arabic
- The FADIC Online Continuous Medical Improvement Programs & Mini-Courses.
- Check Now FADIC Book store and Buy books in different specialties.
- Watch Now FADIC TV to Keep your self Updated.
- FADIC Podcast focusing on varieties of pharmacist perspectives in different specialties.
- Subscribe Now in FADIC 2020 Daily News (FNN) and Keep Updated.
- Check Now about Coronavirus Resource Information Center.

 Log in
Log in Sign up
Sign up