Medication Error Guideline
The Medication Error Guideline

1. Medication Error Guideline
- Medicine errors cause considerable patient morbidity, mortality and increased healthcare costs.
- The published studies estimated that about 5—10% of hospital admissions were due to medication errors.
- It is suspected that approximately 3% of deaths in the Swedish population are because of medication errors.
- In Canada, up to 50% of patient safety incidents in primary care are related to medication errors.
- In the UK, More than 80,000 medication errors occur annually by the National Health Service (NHS) organisations; these errors could cost the NHS more than £750 million6.
- In addition, preventable medication errors cost USA hospitals about $20 billion yearly.
- Medication errors are the fourth most common sentinel event reported in Saudi hospitals.
- One of the main goals of the Ministry of Health is to reduce medication errors and improve patient safety in their institutions by enhancing the medication-use process.
- Reporting medication error is one most effective strategies to improve patient safety.
- While these reports help to understand the medication errors as contributing factors.
- Finally, this guideline was formulated to make reporting medication errors easier to encourage reporting errors with the personal detail confidentially.
The objective of Reporting the Medication error
- Gathering more information about medication errors and maintaining a database to analyse the contributing factors associated with these errors.
- Additionally, when utilising this data to set strategies to reduce medication errors and improve patient safety.
Medication error
- The most commonly used definition is that given by the National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) in the USA, which defines medication errors as:
- “Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, Or consumer; such events music be related to professional practice, healthcare products, procedures, and systems.”
- Note: The Medication Errors include the Intercepted Errors (near-miss).
- Near-miss is defined by WHO “as an error that happened but did not reach the patients. These errors are captured and corrected before the medicine comes to the patient.
- Hazardous Situation is any condition that may lead to a medication error, such as confusion over look-alike/sound-alike drugs or similar packaging or using prohibited abbreviations (Error-Prone).

Medication errors examples:
- Omissions – any prescribed dose not given
- the Wrong dose dispensed or administered – too much or too little
- Extra dose given
- Given the wrong medicine
- Additionally, the Wrong dose interval
- Moreover, the Wrong administration route – administration of a medicine by a different route or in another form from that prescribed
- Wrong time for the administration
- Not following warning advice when administering. Take with or after food.
- Prescribed or administrated a medicine to which the resident has a known allergy
- Dispensed or administrated an expiry date medicine
- Un-prescribed medicine — dispense or administrate authorised medication to a patient.
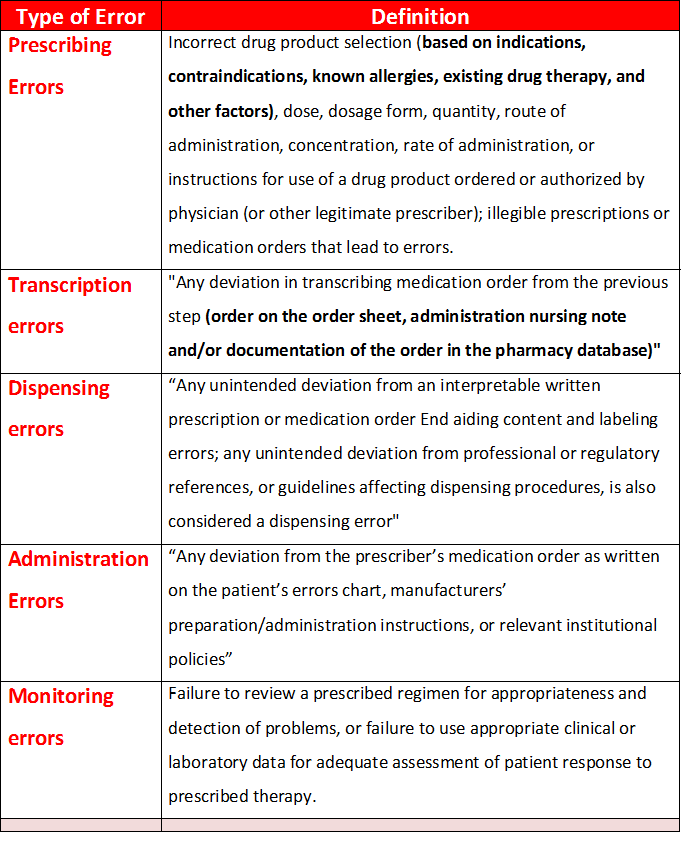
Medication Error Classification
- Errors can be classified according to contextual categories, such as stage of occurrence. So By the medication use process, medication errors can be classified as prescribing errors, transcription errors, dispensing errors, administration errors or monitoring errors.
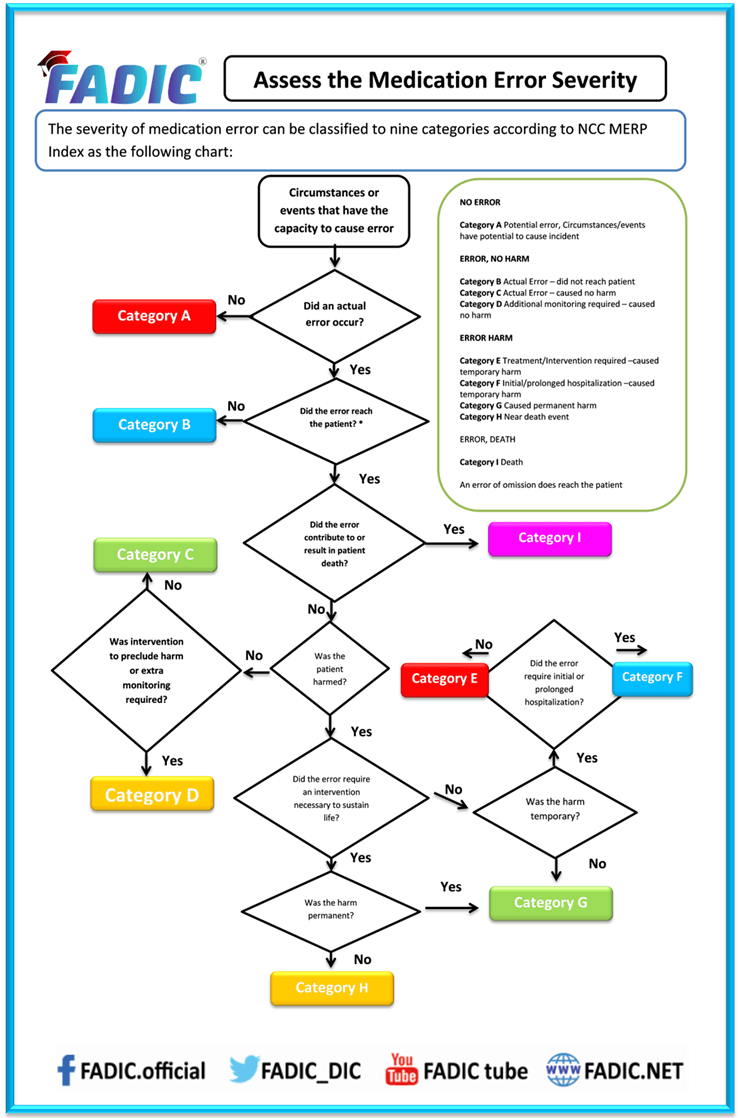
Assess the Medication Error severity:
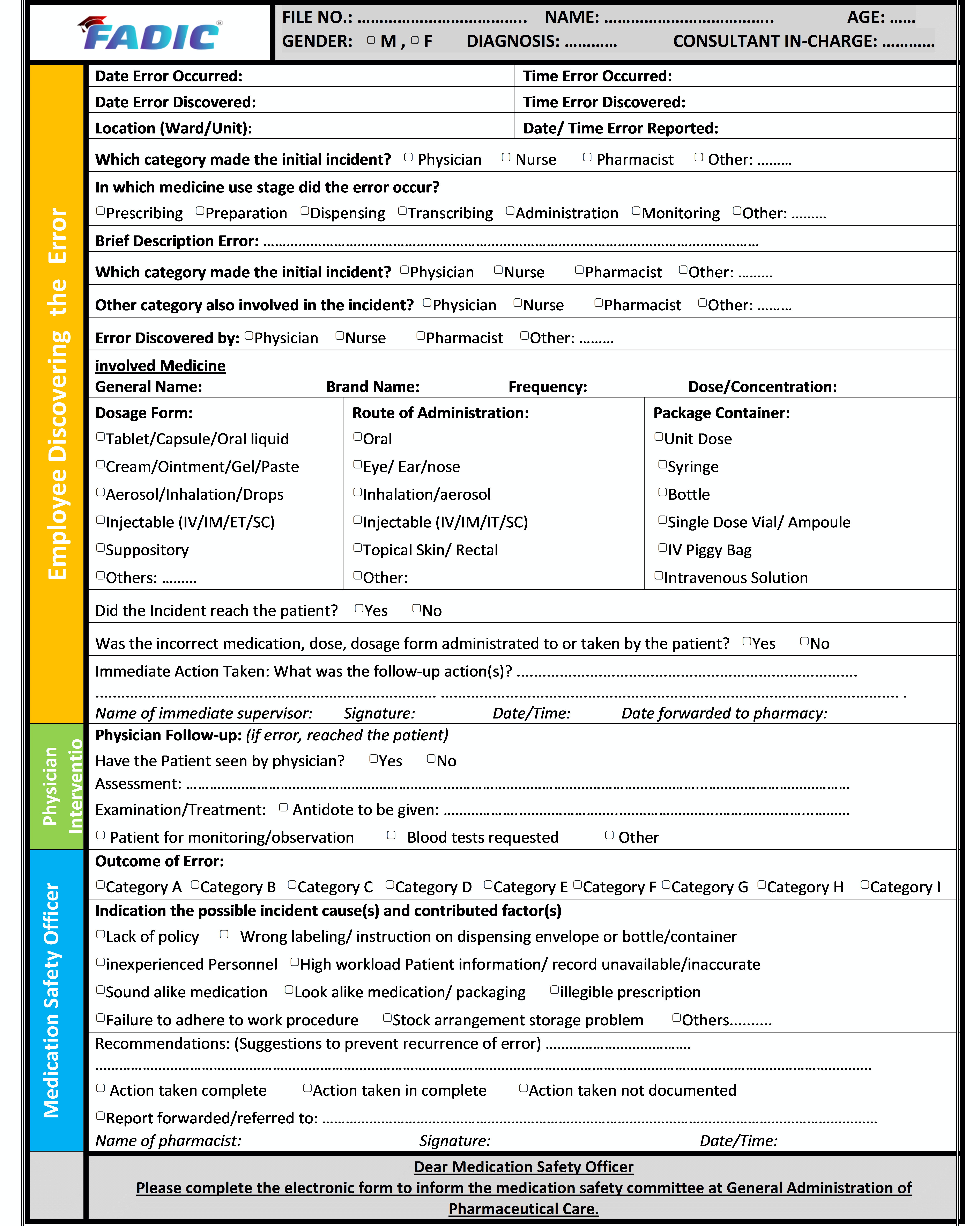
Steps of Reporting Medication Errors
- Any staff member who discovers a medication error, whether a physician, pharmacist or nurse, must immediately complete the Medication Error Report (Appendix I). The details include; patient name, hospital number, prescription details, details of errors and any incorrect medicine or dose administered to the patient.
- When these details of errors are recorded on the form, the manager or deputy needs to identify those staff involved, explain the error to get them, and then write about the causes of any comments about the error. The manager or deputy needs to mention the immediate action taken.
- Send the completed form to the Pharmacy department in the hospital within 24 hours
- The Medication Safety Officer must complete the medication error, such as assessing the incident severity, conducting Root Cause Analysis (RCA) if needed (for all significant or potentially significant medication errors) and suggesting recommendations to reduce the reoccurrence of the error.
- The Medication Safety Officer need to inform the Medication Safety Committee by completing the electronic report form through the following link: http://www.research.net/r/LG2HKB8
- Medication Safety officers in the hospital must review all the medication errors and take the necessary action to avoid occurring similar mistakes in the future.
- Forwarded to the hospital’s Total Quality Management (TQM) Department.
2. Guideline on Safe Use of High Alert Medications
- Hospitals and healthcare providers aim to provide high-quality and safe medical care to their patients, including the safe and effective use of medications.
- These medications, however, can be compared to a two-edged sword.
- While useful, they can also be harmful due to errors associated with their use and adverse events/effects.
- Especially with these medications that have a very narrow margin of safety and can cause severe harm to the patient.
- Additionally, these medications are recognised as High Alert Medications.
- The Institute for Safe Medication Practices has gathered a list of “high-alert” medications.
- Finally, these medications require extra precaution because they have highly potentially rich to the patient when used in error.
Objectives
- These guidelines aim to clarify the medicines category considered to be high-alert status and outline the various strategies for safeguarding the use of high-Alert Medications to improve patient safety.
Definition
- High-Alert Medications are medicines with a high potential risk to the patient when utilised in error. Although mistakes may or may not be familiar to these medicines, an error’s significance is more devastating to patients.
High Alert Medication Category
- Based on error reports submitted to the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program, reports of harmful errors in the literature, and input from practitioners and safety experts, ISMP created and periodically updated a list of potential high-alert medications.
- The Medication Safety Officer must edit this list in the hospital based on the available medicines and localised medication error reports.
A- High Alert Medications Classes/ Categories List
- adrenergic agonists. IV (e.g.. EPINEPHrine, Norepinephrine. Phenylephrine)
- adrenergic antagonists. IV (e.g., Bisoprolol. Propranolol, Labetalol)
- anaesthetic agents, general, inhaled, and IV (e.g., Propofol, Ketamine)
- antiarrhythmics, IV (e.g., Lidocaine, Amiodarone, Adenosine)
- antithrombotic agents (e.g., Warfarin, Heparin. Streptokinase)
- cardioplegic solutions
- chemotherapeutic agents, parenteral and oral
- dextrose, hypertonic. 20% or greater
- dialysis solutions, peritoneal and hemodialysis
- epidural or intrathecal medications
- Oral Hypoglycemic: (e.g., Glibenclamide, Gliclazide, Metformin Hcl)
- inotropic medications, IV: (e.g., Digoxin, Milrinone, lnamrinone)
- insulin, subcutaneous and IV
- liposomal forms of drugs (e.g., liposomal amphotericin B) and conventional counterparts (e.g., amphotericin B desoxycholate)
- moderate sedation agents, IV, oral, for children: (e.g., dexmedetomidine, midazolam)
- Narcotics/opioids, IV, transdermal and oral (including liquid concentrates, immediate and sustained release formulations)
- neuromuscular blocking agents: (e.g.. Suxamethonium. Rocuronium, Vecuronium)
- parenteral nutrition preparations
- radiocontrast agents, IV
- sterile water for injection
- sodium chloride for injection, hypertonic, more significant than 0.9% concentration
B-Specific High Alert Medications List
- Colchicine injection
- Epoprostenol, IV
- Insulin, subcutaneous and IV
- Magnesium Sulfate injection
- Methotrexate, oral, for non-oncologic use
- Oxytocin, IV
- Nitroprusside Sodium for injection
- Potassium Chloride for injection concentrate
- Potassium Phosphates injection
- Promethazine, IV
- Sodium Chloride injection, hypertonic (greater than 0.9% concentration)
- Sterile Water for injection. inhalation and irrigation (excluding pour bottles) in containers of 100 mL or more
Managing High Alert Medications
- The pharmacy department in the hospital needs to provide general guidelines for the proper handling of High Alert Medications, including the medication list, by the CBAHI Standards
- Concentrated electrolytes (Potassium & Sodium Phosphate, Potassium Chloride, and Sodium Chloride) are High-Alert Medications.
- So, they should not be stocked in the patient care areas except as part of the crash cart medications; limited quantities of these concentrated electrolytes that stored in specific areas such as ICU and ER and need to be kept in a separate locker and away from the regular ward stock medications and require monitoring frequently by nursing and pharmacy staff
- Label all containers and shelves used for storing High Alert Medications as “HIGH ALERT MEDICATION.”
- High Alert Medications must have double-checked before they are prepared, dispensed and administered to the patients
- The Medication Safety officer in the hospital must check if the staff is committed to double-checking before being prepared, dispensed and administered to the patients.
Strategies to reduce errors involving High Alert Medication
Procurement
-
- Limit the drug strengths available in the hospital.
- Avoid frequent changes of brand or colour and notify the other healthcare staff if there are changes.
- Inform all relevant personnel in the hospital about the new High Alert Medications listed.
Storage
-
-
- Minimize High Alert Medications from clinical areas, where possible.
- High Alert Medication should be stored individually in a labelled plastic container.
- Label the shelves or containers used for storing High Alert Medications as HIGH ALERT MEDICATION”.
-
Prescribing
-
- Avoid using abbreviations when prescribing High Alert Medications.
- Avoid ordering High Alert Medications verbally accepted in case of emergency orders have to repeat and verified.
- Prescribe oral liquid medications with the dose specified in milligrams.
- Avoid using trailing zero when prescribing. (e.g. 5.0 mg can be mistaken as 50 mg)
- Reduce the total dose of High Alert Medications in continuous IV drip bags (e.g., 12,500 Units of Heparin in 250 ml vs 25,000 Units in 500 ml) to reduce risk.
Dispensing / Supply
-
- All High Alert Medication containers, product packages, vials or ampoules issued towards/units need the caution label “HIGH ALERT MEDICA11ON’ except for parenteral nutrition preparations.
- You can not dispense High Alert Medications to patients without cautioning label
- Accuracy check performance must be applied for the High Alert Medications before dispensing the medicines.
Counselling
-
- Educate the patient and, or the patient family member and the required information, such as; the purpose of taking medicine, how to take medicine and the common side effect of using the medicines
Administration
-
- Nurses must double-check all High-Alert medications before giving the dose to the patient.
- In addition, the following particulars shall be independently double-checked against the prescription or medication chart at the bedside by two appropriate persons before administration
- Patient’s name and patient fill number
- Medication name, strength and dose
- Route and rate (pump setting and line placements when necessary)
- Expiry date
- Ensure trained personnel do administration of intrathecal, cytotoxic drugs, epidural analgesics and parenteral nutrition.
- Return all unused or specially formulated preparations to the pharmacy when no longer required.
Monitoring
-
- Closely monitor vital signs, laboratory data, and patient’s response before and after administering High Alert Medications.
- Keep antidotes and resuscitation equipment inwards) units.
3. Guide on handling look-Alike & Sound-Alike Medications
- The patient safety incidents are widely spread because the health services system has become more complex due to new technologies, medicines and treatment strategies.
- Currently, thousands of medications are available in the markets and hospitals.
- Additionally, some of these medicines have similar names or packaging.
- The evidence shows that the names and packaging of Look-alike/sound-alike medicines are among the most common factors associated with medication errors.
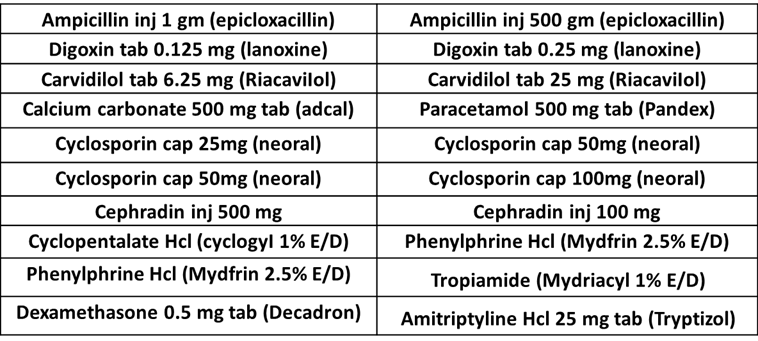
- Look Alike Sound Alike (LASA) medications involve medicines that are visually similar in appearance or packaging and names of drugs with spelling similarities and/or similar phonetics.
- Appendix 1 shows a list of medications that have a similarity in names. The pharmacy department in the hospital needs to update the LASA medications list every year.
Objectives
- This guideline aims to prevent potentially harmful medication errors that may result from confusing medication names and similar product packaging and to guide the healthcare providers and institutes to maintain a good to reduce the medication errors.
Contributing Factors
Several Contributing factors may lead to confusion with LASA medications, these includes
- Illegible handwriting.
- Incomplete knowledge of drug names.
- Newly available products.
- Importantly, it has Similar packaging or labelling.
- Similar strengths, dosage forms, and frequency of administration.
- Finally, Similar clinical use
Strategies to avoid errors with LASA Medications
-
-
Procurement:
-
- Minimize the availability of multiple medicines strengths
- Whenever possible, avoid purchasing medicines with similar packaging and appearance. As new products or packages are introduced, compare them with existing packaging.
-
-
Storage:
-
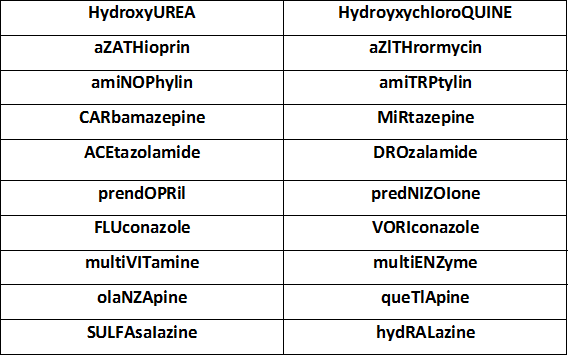
- Use Tall Man lettering to emphasise differences in medications with sound-alike names.
- Tall Man lettering (or Tallman lettering) is the practice of writing part of a medicine’s name in upper case letters.
- In addition, it helps distinguish sound-alike/look-alike medications from one another to avoid medication errors.
- Examples of Tall Man lettering are metFORMIN and metoPROLOL
- Using caution red tag notes on shelves to alert the dispenser that medicine has look-alike and sound-alike medicines.
- Additionally, using techniques such as boldface and differences reduces the confusion associated with using LASA names on labels in the medicine’s storage containers and shelves.
-
-
Prescribing:
-
- Place LASA medications in locations separate from each other or in non-alphabetical order. (a) Write legibly, using the brand and generic names for prescribing LASA medications.
- Prescription should specify the name of the medication, dosage form, dose and complete direction for use.
- Write the diagnosis or medication’s indication for use.
- In addition, this information helps to differentiate possible choices in illegible orders.
- In the electronic prescribing system, using techniques such as Tall-man lettering.
- Additionally, using the boldface and colour differences reduces the confusion associated with using LASA names on computer screens and medication administration records.
- Communicate clearly.
- Take time pronouncing the drug name whenever an oral order is made.
- Ask the recipient of the oral communication to repeat the medication name and dose.
- Minimize the use of Verbal and Telephone orders.
-
-
Dispensing/Supply:
-
- Identify medicines based on their name and strength, not their appearance or location.
- Check the purpose of the medication and the dose for the medicines dispensed.
- Read medication prescription and label carefully at all dispensing stages
- Commitment to a final accuracy check by a qualified person before handing over the medicine to the patient or the patient’s representative
- Double-check should be conducted during the dispensing and supply process.
- Highlight changes in medication appearances to patients upon dispensing.
Administration:
- Read carefully the medication labels each time during the administration process
- Perform a double-check to check the actual medicine and compare it with the prescription and label.
- Check the purpose of the medication and the dose before administration.
Monitoring:
- Monitor new drugs added to the hospital formulary as they are released and provide guidelines for these new drugs.
- Monitor patients who may have received wrong medications due to LASA medication errors.
Information:
- Update healthcare professionals on changes on the list of LASA and confusing drug names.
- Provide education on LASA medications to healthcare professionals at orientation and as part of continuing education.
Patient Education:
- Inform patients about changes in medication appearances.
- Educate patients and their caregivers to alert healthcare providers whenever a medication appears to vary from what is usually taken or administered.
- Encourage patients and their caregivers to learn the names of their medications.
Evaluation
- Evaluate medication errors related to LASA medications.
Sound ALIKE Medications
For More Sound ALIKE Medications From FADIC, from this LINK
LOOK ALIKE Medications
For More LOOK ALIKE Medications From FADIC, from this LINK
Read More
- Antimicrobial Stewardship School
- Medication Safety Online Course
- Medication Errors: How to Avoid? Mini-Course
- Download Pocket Guide for Antibiotic Pharmacotherapy Book
- Pharmacovigilance & Drug Safety Workshop
- Medication Safety Course Certification (Arabic Version)
- Medication Error Guideline
- List of Medication with Black Box Warning

 Log in
Log in Sign up
Sign up