Why Take this Fellowship?
FADIC Drug Information Fellowship | 12-months | Accredited by 100 CME Hours | Dynamics | FADIC Center for Drug Information & Evidence-Based Practice
Overview
Drug Information Fellowship Course
Online Course | 12 Months | Weekly Basis
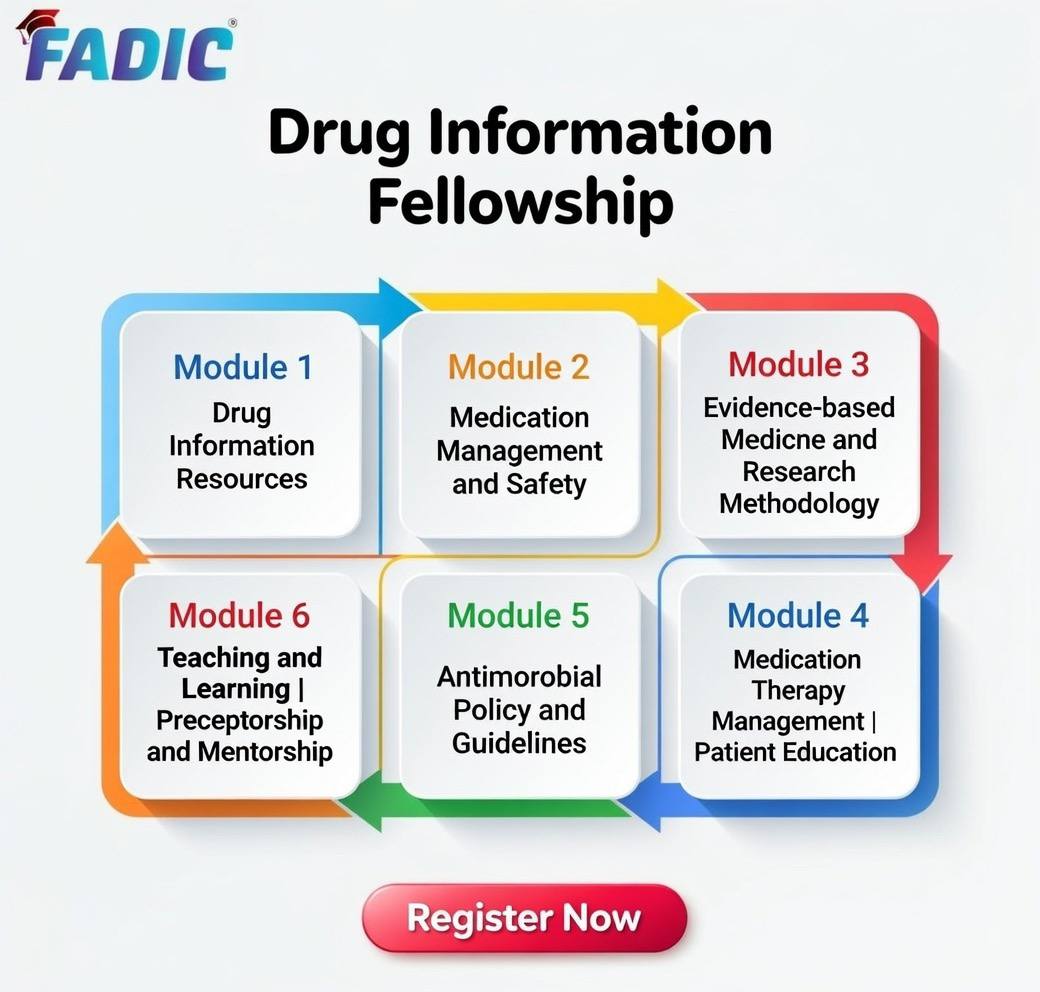
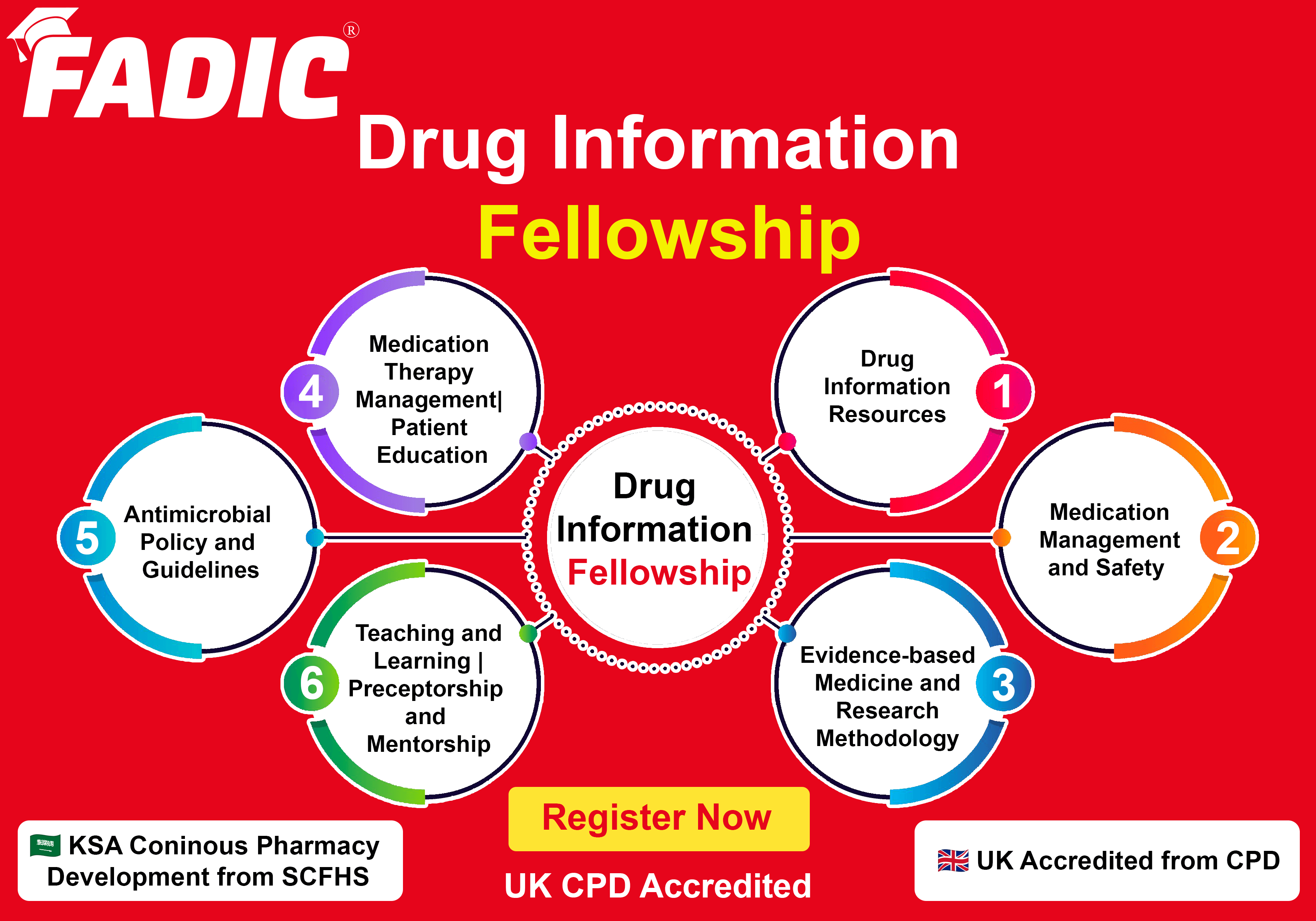
Drug Information Fellowship Modules:
(100 CME Hours)
- Module 1: Drug Information Resources: 8 Weeks
- Module 2: Medication Management and Safety: 8 Weeks
- Module 3: Evidence-based Medicine and Research Methodology: 8 Weeks
- Module 4: Medication Therapy Management| Patient Education: 9 Weeks
- Module 5: Antimicrobial Policy and Guidelines: 9 Weeks
- Module 6: Teaching and Learning | Preceptorship and Mentorship: 8 Weeks
Fellowship Accreditations
- FADIC is UK training provider in the United Kingdom (UK).
- The Drug Information Fellowship is Accredited by FADIC.
- This Fellowship is Compatible with the SCFHS requirements in Saudi Arabia.
- It is a 12-month program designed to prepare the pharmacy professional interested in a drug information/medical information career.
- Upon your enrollment, it is mandatory to complete all the modules of the fellowship within a two-year timeframe from the date of your registration.
Why Drug Information Fellowship?
- Pharmacists are the most accessible medical professionals for the general public and the medical community.
- They are the first to be contacted for drug information.
- Drug information needs range from simple drug-specific requirements to complex patient-specific treatments.
- Providing accurate drug information at these various levels requires developing skills related to collecting, storing, acquiring, interpreting, and distributing drug information as a first step.
- Relevant scientific and practical training in drug information, medication management, clinical research, patient counselling and clinical pharmacies are the basic requirements of training to become a pharmacist specialist in drug informatics.
- All pharmacists are related to drug use, side effects, tolerability, etc.
- You need to be able to use essential pharmacological texts to answer various drug information questions.
What Drug Information Fellowship
- The drug information fellow will be at an academic drug information centre.
- Moreover, throughout the program, the fellow will develop advanced skills in medical/drug information practice as a drug information specialist in various practice settings.
- The program also includes opportunities to gain experiences in pharmacy education/academia through teaching and precepting experiences at the pharmacy practice.
- Finally, in the process, the fellow will also have the option to obtain a teaching certificate.
Objectives / Purpose:
- This program is designed to develop an advanced practitioner in providing medical information and policy development.
- Provides opportunities to participate in numerous activities.
- All these activities enable the medical information specialist to encounter an industrial and hospital setting and diverse clinical exposures in pharmacy specialities.
- In addition, it will work with established pharmacies and clinicians on interdisciplinary teams throughout the hospital and community settings.
- You will spend six months training at the FADIC Drug Information, pharmacy journal club, medication management, evidence-based medicine, research and six months with medication therapy management and patient-centred areas.
- Moreover, upon completing this program, you will have the experience and clinical skills necessary to practice as a drug information specialist in various settings.
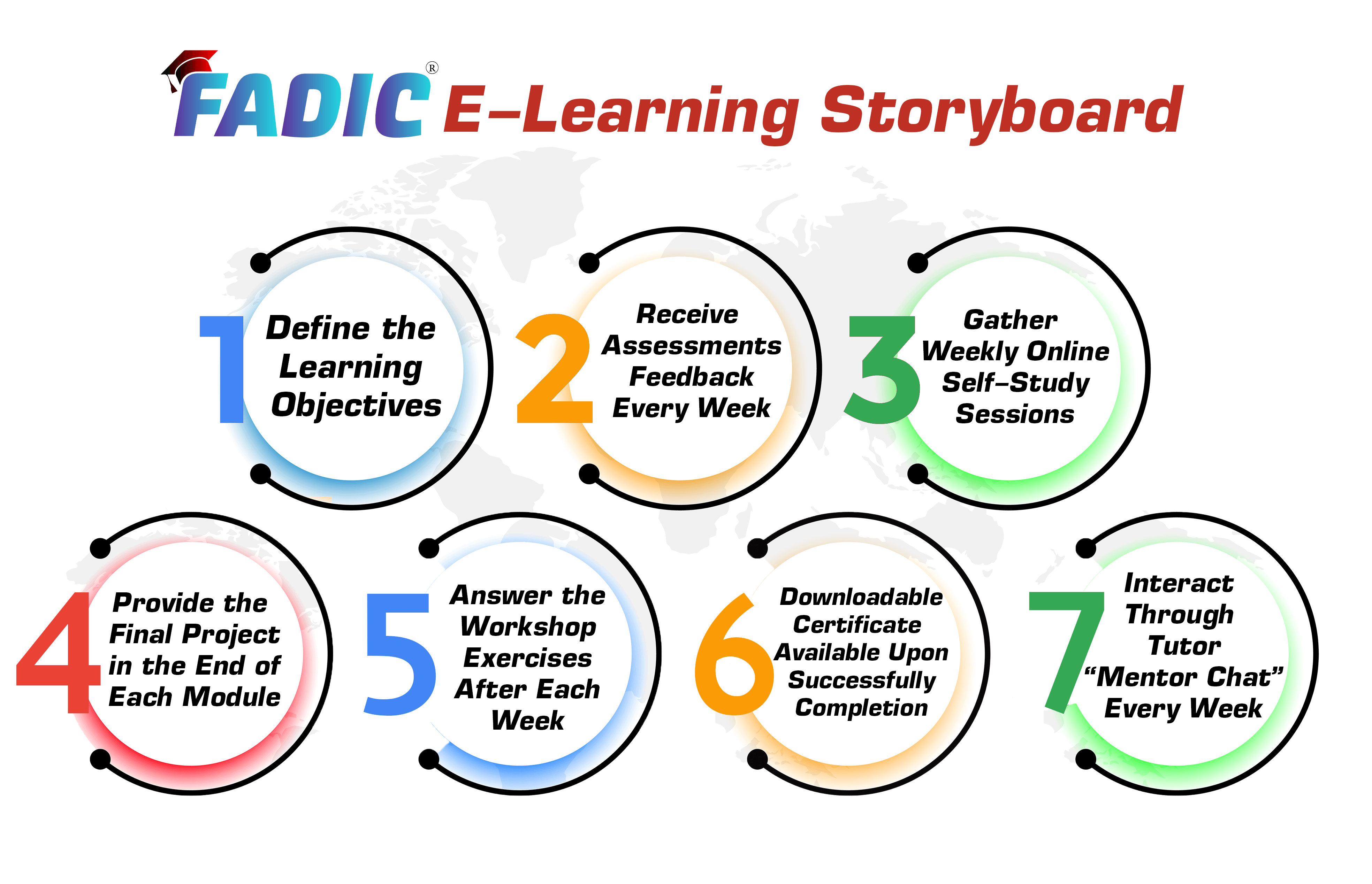
| What You Will Get Every Week (Lesson Planning) | |||
| Online Lesson | Workshop / Project | Mentor Support | Reviewer |
| (2-3 Hours) per week | (60 Minutes) per week | Every week | Review the answers with feedback |
About the Fellowship Program
- Firstly, FADIC offers a dynamic 1-year research fellowship program in drug information with an emphasis on evidence-based practice.
- Additionally, the program offers an intensive, rewarding experience in drug information-related research.
- Moreover, develop the clinical practice that will build your skills.
- It is suitable for a career path in academia, industry, or clinical practice.
- Finally, the program provides an intensive experience in drug information-related research, practice, teaching and learning.
Purpose
- The purpose of this fellowship Program is to prepare you to practice as a drug information specialist.
- Moreover, practice excellence is achieved by:
- Demonstrating accountability for focused project work
- Modelling excellence in drug information practice
- Participating in research and performance improvement, projects focused on clinical care and improving the medication use process.
- Demonstrating the ability to collaborate with healthcare professionals to communicate critical information and guide decision making
- Facilitating, leading, and actively participating in discussions and committees necessary to the role of a hospital and industry drug information practitioner
- Serving as a professional role model for others in training
Outcomes
Required Outcomes
- Firstly, serve as an authoritative resource on the formulary management process.
- Moreover, guide the development of recommendations that serve to optimise a health system formulary.
- Provide evidence-based, patient-centred medication therapy recommendations during drug information consults.
- Additionally, make recommendations to optimise the use of medications by participating in the medication use evaluation.
- Make recommendations to optimise drug information content in promotional and content writing.
- Participate in activities that drive the development of action plans during medication management.
- Moreover, participate in the development of medication policy, guidelines and strategic plan.
- Demonstrate excellence in providing training or educational activities for healthcare professionals in interprofessional training.
- Actively participate in the analysis and investigation of adverse drug event reporting and medication errors
- Complete required rotations, word rounds and clinical work
- Conduct drug information and pharmacy practice research
- Function effectively in hospital, ambulatory, and community.
Elective Outcomes
- Publish on topics related to the pharmacy or drug information
Application Process
- The Drug Information Fellowship Application Process.
- Additionally, the application should include a letter of interest, curriculum vitae, and two letters of recommendation.
- Moreover, the application materials for FADIC in the Drug Information Fellowship must be sent electronically.
- Applications should be sent to the following e-mail address: Info@FADIC.net
Topic Covered in Drug Information Fellowship
- First: Drug Information | Electronic Resources | Library | Journals
- Second: EBM | Researcher | Scholarship | Publications | Systematic Reviews
- Third: Medication Management and Use | Medication Safety | Risk Management | Pharmacoeconomics
- Fourth: Pharmacist as a prescriber | Counseling | Medication Therapy Management | Vitamins
- Fifth: Educator | Preceptor | Teaching Philosophy | Clinical Pharmacy Pathway | Mentorship | Leadership
Fellow Qualifications
- The fellow should hold a Bachelor’s Degree, Pharm D, BPS, Residency, or Master’s Degree in Pharmacy before starting the program and be eligible for the fellowship.
- In addition, previous pharmacy work experience or a residency in pharmacy practice is preferred but not required.
- Demonstrated ability to communicate proficiently in writing and verbally is required.
- The fellow must be a self-starter and have the ability to manage numerous projects and responsibilities.
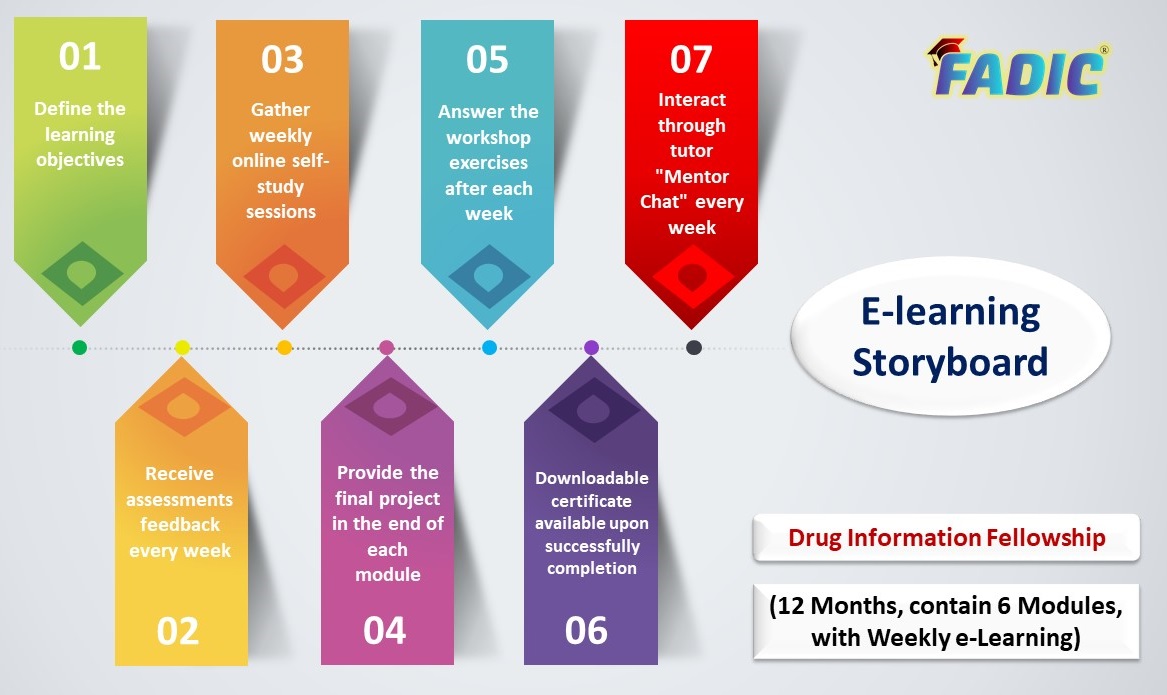
Fellowship E-Learning Storyboard
Drug Information Fellowship offers Three core experiences:
- Drug information clinical practice, journal club review, systematic review and research
- Medication Therapy Management, patient-centred care
- teaching and learning, preceptorship
Drug Information Consultation Service.
- The fellow will become a competent practitioner in drug information clinical practice.
- Additionally, by participating in the day-to-day operations and management of the drug information consultation service.
- Moreover, understand statistical analysis, clinical trials, and writing articles and research papers
- This is a core competency emphasised throughout the program’s duration.
- A thorough understanding and skill in literature evaluation, literature reviews, systematic reviews and evidence-based practice principles is required for this experience.
Research
- The fellow will spend up to 75% of their time in research, medication information, diseases, or literature search.
- Additionally, the fellow will work collaboratively on research conducted through the FADIC centre for Drug Information & Evidence-Based Practice.
- A variety of research opportunities are available in the areas of clinical practice in drug information, scholarship of teaching and learning, meta-analysis and systematic reviews, and others.
- The fellow may also have the opportunity to work with researchers outside the Center.
- Finally, the fellow will gain experience developing grant proposals, research proposals, project planning and implementation, data collection and analysis, and development of scholarly manuscripts.
Medication Policy /Guidelines/Committee Support.
- The fellow will have the opportunity to be involved in the committee support work with affiliated institutions and other organisations.
Teaching & Education.
- The fellow will receive an academic appointment as an Instructor of Pharmacy Practice in the FADIC lecturer system.
- In addition, the fellow will learn how to provide extensive advanced experiential training and mentorship to the pharmacy students during their required drug information fellowship and opportunities to teach in a didactic setting.
Elective Opportunities.
- The fellow is encouraged to explore areas of interest by completing projects that focus on areas of interest.
Training Programs / Certificates / Teaching Certificate.
- The fellow will participate in the required teaching training sessions.
- This longitudinal program will help develop foundational curriculum development and teaching skills.
Evidence-Based Practice Certificate.
- The fellow will complete the required evidence-based practice certificate through the FADIC Center for Drug Information & Evidence-Based Practice.
- Additionally, the program intended to provide the foundational knowledge for evidence-based medical decision-making, including modules covering biostatistics, hypothesis testing, research design, developing a research protocol, and conducting a systematic review and meta-analysis.
Fellowship Website
- The fellowship program will be online through the FADIC Website.
- Duration: 12 months
How to Apply
- Submit by email:
– A letter of intent
– CV
– Two (2) letters of recommendation
– Pharmacy degree transcripts
to: Info@FADIC.net
With FADIC Drug Information Fellowship, you Will Have…
FADIC Center for Drug Information & Evidence-Based Practice
Enhance your learning with the FADIC Mentorship Reviewer System. A dedicated mentor will guide you through your projects and workshops, providing personalized feedback to help you succeed.
Maximize your learning with downloadable and printable FADIC handouts. Study effectively and independently at your own pace.
Stay Ahead with FADIC: 36-Month Access to All Updates and Course Materials
Stay updated with FADIC: Starting from your registration date, you will enjoy 36 months of full access to all course materials, including the latest updates and course content.

You will get FADIC Drug Information Flash Cards Book For Free after Registration

Finally, Get your Downloadable Certificate
The holding of this FADIC Drug Information Fellowship Certificate (100 CME Hours) is intended to enhance competence but not replace or act as a primary qualification.
Includes 6 Modules
Module 1:
Drug Information Resources (8 Weeks)
Module 2:
Medication Management and Safety (8 Weeks)
Module 3:
Evidence-based Medicine and Research Methodology (8 Weeks)
Module 4:
Medication Therapy Management| Patient Education (16 Weeks)
Module 5:
Antimicrobial Policy and Guidlines
Module 6:
Teaching and Learning | Preceptorship and Mentorship (8 Weeks)
Meet the Lecturer
Dr. Rasha Abdelsalam

Dr. Rasha is a CEO of FADIC. She was in Tanta university hospital, Egypt. In 2013, she received TQM from American university, BCPS, CPHQ, and M.Sc. of Clinical Pharmacy. She received certification from SIDP. She obtained the add qualification from American board in infectious disease. Leaded MM CBAHI Saudi accreditation. Established stewardship committee, and implementation in MCH, Makkah, Saudi Arabia.
She shared in item exam writing for BCIDP Exam. She shared as a reviewer in infectious diseases PSAP. Authorship for published around 20 papers. One paper about vision of 2030 of general pharmaceutical department in Saudi Arabia. She received TLC certification from ACCP academy. Moreover, consultant on personal, professional, stewardship leadership, research. Share in strategic planning; hospital accreditation; and stewardship education.
Related Courses:
- Drug Information Program and Workshop
- Biostatistics Clinical Guide
- Interpretation of Clinical Trials
- Medication Errors: How to Avoid?
- Systematic Review Course
- Journal Club Presentations Course
- Applied Medical Resources
- FADIC Pharmacokinetics Program
- The Future Guide for Pharmacist
Hashtags
1. # fellowship, fellowship programs
2. #pharmacist fellowship, fellowship pharmacy
3. #fellowship in pharmacy, drug information fellowship
4. #pharmacy fellowship programs
5. #clinical pharmacy fellowship, fellowship meaning, what is a fellowship
6. #pharmacy fellowship programs, research fellowship
If you have any inquiry, please contact WhatsApp.

Reviews
البرنامج مفيد جدا وتأسيسي ويلم بكل ادوار الصيدلي في مركز معلومات الأدوية
Amal Awad Aبرنامج الزماله في معلومات الدواء يعتبر من اقوي البرامج الموجوده حيث يتميز بسهولة الطرح والشرح ويغطي جميع المواضيع المتعلقه بمصادر معلومات الدواء المختلفة وطريقة ايجاد الاجابة علي الاسئله السريريه المعقدة ويغطي مواضيع السلامة الدوائية وغيرها من المواضيع ذات العلاقة والتي من المهم لكل صيدلي تعلمها والحصول عليها
Majed Alorabi
 Log in
Log in Sign up
Sign up